Question: ed: Jan 11 at 9:48pm uiz Instructions > Question 17 If 34.50 mL of 0.25 M NaOH is needed to titrate 10.00 mL of a
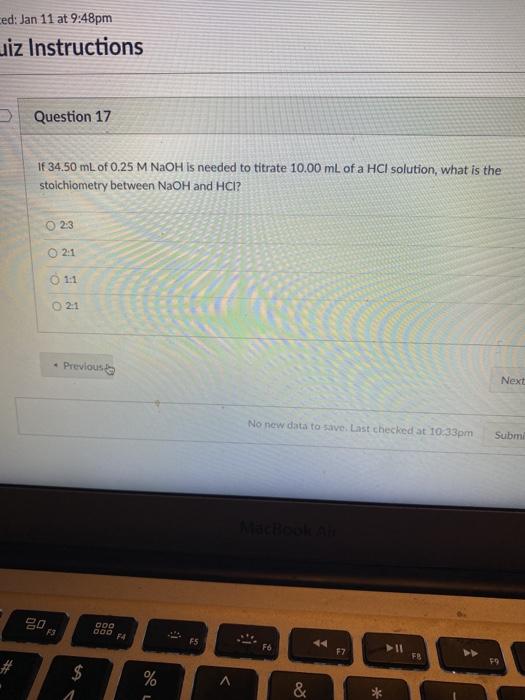
ed: Jan 11 at 9:48pm uiz Instructions > Question 17 If 34.50 mL of 0.25 M NaOH is needed to titrate 10.00 mL of a HCl solution, what is the stoichiometry between NaOH and HCI? 2:3 02:1 11 2:1 Previous Next No new data to save. Last checked at 10.33pm Subm 20 F3 DOO 000 F F6 17 FB $ 4 % *
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


