Question: EECE 503 1. Using simple collision theory estimate the collision number for 1 mol (6.022 x 102 molecules) of hydrogen iodide (HI) present in a
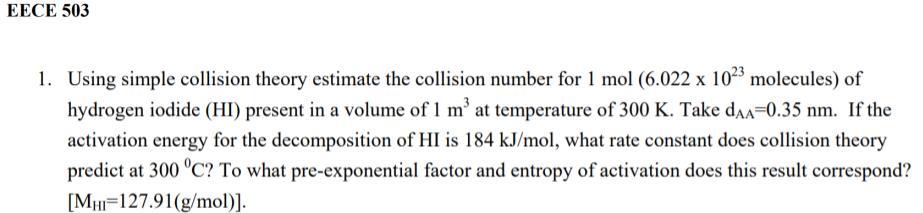
EECE 503 1. Using simple collision theory estimate the collision number for 1 mol (6.022 x 102 molecules) of hydrogen iodide (HI) present in a volume of 1 m at temperature of 300 K. Take daa=0.35 nm. If the activation energy for the decomposition of HI is 184 kJ/mol, what rate constant does collision theory predict at 300 C? To what pre-exponential factor and entropy of activation does this result correspond? [Mw=127.91(g/mol)]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


