Question: EHMEB 2 A Homework Exercise Due: 0 3 April 2 0 2 4 Sulphur dioxide may be converted to S O 3 , which has
EHMEBA Homework Exercise
Due: April
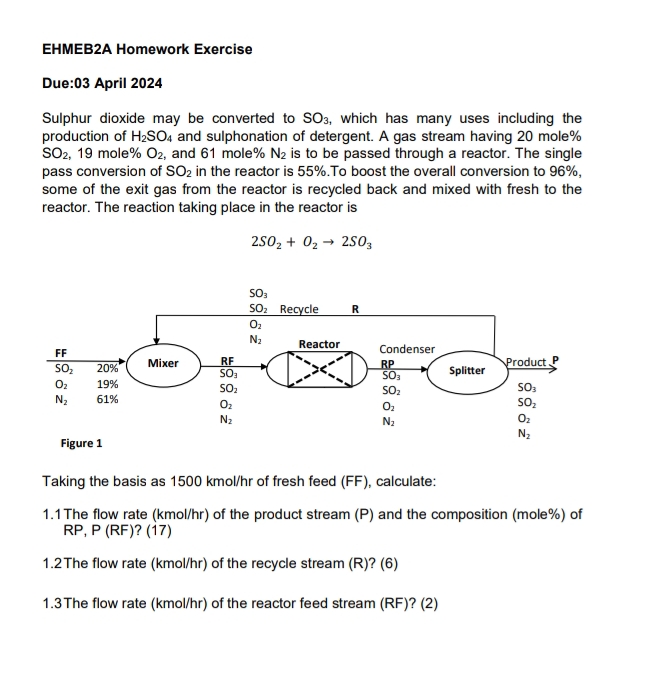
Sulphur dioxide may be converted to which has many uses including the production of and sulphonation of detergent. A gas stream having mole mole and mole is to be passed through a reactor. The single pass conversion of in the reactor is To boost the overall conversion to some of the exit gas from the reactor is recycled back and mixed with fresh to the reactor. The reaction taking place in the reactor is
Taking the basis as kmo of fresh feed FF calculate:
The flow rate of the product stream and the composition mole of RP P RF
The flow rate of the recycle stream R
The flow rate kmo of the reactor feed stream RF
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


