Question: electochemistry please answer the Questions Solution The given values are as follows: 0 8.7 3 8 6 7.1 9 6 12 5.2 15 4.4 18
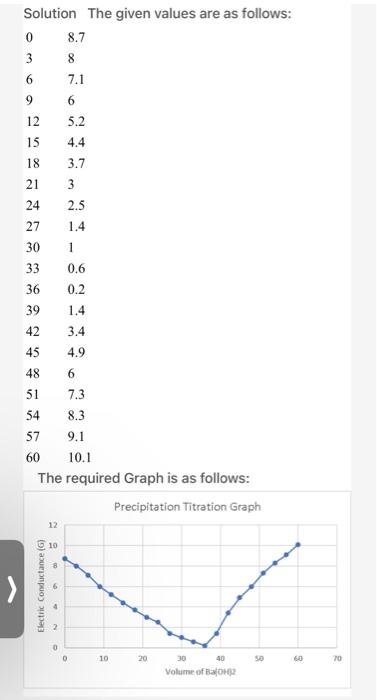
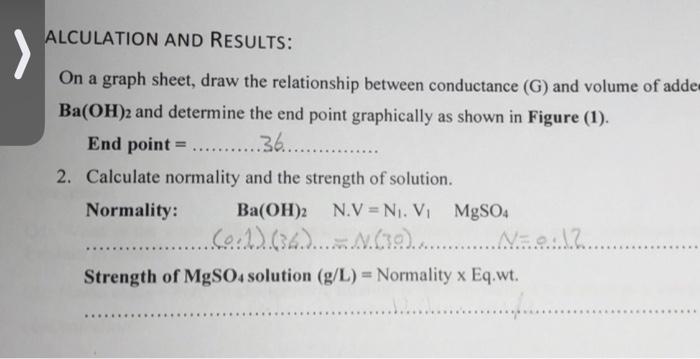
Solution The given values are as follows: 0 8.7 3 8 6 7.1 9 6 12 5.2 15 4.4 18 3.7 21 3 24 2.5 27 1.4 30 1 33 36 0.2 39 1.4 42 3.4 45 4.9 48 6 51 7.3 54 8.3 57 9.1 60 10.1 The required Graph is as follows: Precipitation Titration Graph 0.6 12 10 > Electric Conductance (6) 0 10 20 50 20 30 40 Volume of BOH ALCULATION AND RESULTS: ... On a graph sheet, draw the relationship between conductance (G) and volume of adde Ba(OH)2 and determine the end point graphically as shown in Figure (1) End point = .36.............. 2. Calculate normality and the strength of solution. Normality: Ba(OH)2 N.V=N. VI MgSO4 .Co.1) 634.)...............Naaa......... Strength of MgSO4 solution (g/L) = Normality x Eq.wt
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


