Question: electrochem options are (select all that apply ) : A The ratio between the concentrations of F+ and Fc at the electrode surface is governed
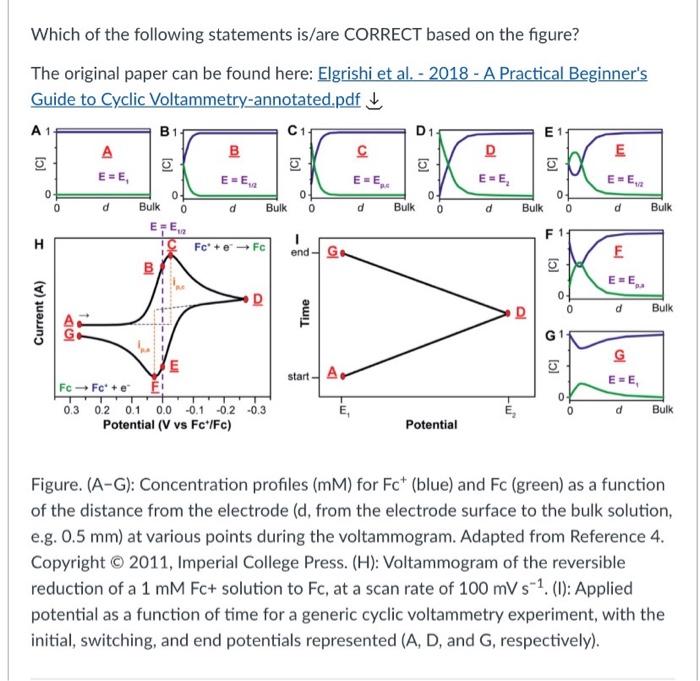
Which of the following statements is/are CORRECT based on the figure? The original paper can be found here: Elgrishi et al. - 2018 - A Practical Beginner's Guide to Cyclic Voltammetry-annotated.pdf 5 A 1 B1 C1 D1 E B D E E=E, E=E, D E E [C] [C] [C] [C] [o] EEE EE E-E 0 0 0 0 0 d d 0 0 Bulk d 0 0 Bulk d Bulk d Bulk 0 d Bulk E, F F H IC Fc + e-Fc end G. E 100 (C) EE o 0 0 d Bulk Current (A) Time D start A A E=E E Fc Fc + E! 0.3 0.2 0.1 0.0 -0.1 0.2 -0.3 Potential (V vs Fc+/Fc) E TE , 0 Bulk Potential Figure. (A-G): Concentration profiles (MM) for Fc (blue) and Fc (green) as a function of the distance from the electrode (d, from the electrode surface to the bulk solution, e.g. 0.5 mm) at various points during the voltammogram. Adapted from Reference 4. Copyright 2011, Imperial College Press. (H): Voltammogram of the reversible reduction of a 1 mM Fc+ solution to Fc, at a scan rate of 100 mV s-1. (1): Applied potential as a function of time for a generic cyclic voltammetry experiment, with the initial, switching, and end potentials represented (A, D, and G, respectively)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


