Question: Electron Configuration Worksheet Circle the correct answer for problems #1-7 d)/=1 d) + Problem 1: s orbital Problem 2:p orbital Problem 3:n Problem 4: angular
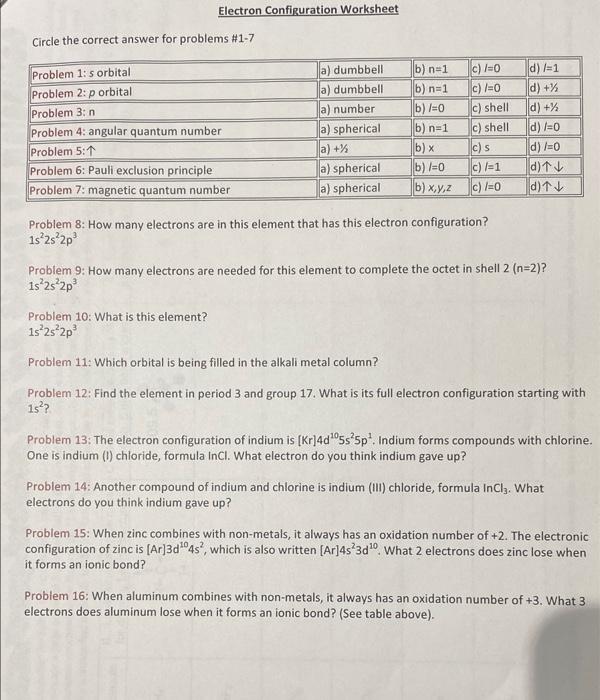
Electron Configuration Worksheet Circle the correct answer for problems #1-7 d)/=1 d) + Problem 1: s orbital Problem 2:p orbital Problem 3:n Problem 4: angular quantum number Problem 5:1 Problem 6: Pauli exclusion principle Problem 7: magnetic quantum number d)+% a) dumbbell a) dumbbell a) number a) spherical a) +% a) spherical a) spherical b) n=1 b) n=1 b)x=0 b) n=1 c) 1=0 c)/=0 c) shell c) shell c)s c) /=1 (b)x d) /=0 d)/=0 (d) tv d) 1 b)/=0 (b) x, y, z C)/=0 Problem 8: How many electrons are in this element that has this electron configuration? 1s 2s 2p Problem 9: How many electrons are needed for this element to complete the octet in shell 2 (n=2)? 1s 2s 2p Problem 10: What is this element? 1s 2s 2p Problem 11: Which orbital is being filled in the alkali metal column? Problem 12: Find the element in period 3 and group 17. What is its full electron configuration starting with 1s?? Problem 13: The electron configuration of indium is [Kr]4d05s 5p. Indium forms compounds with chlorine. One is indium (1) chloride, formula InCI. What electron do you think indium gave up? Problem 14: Another compound of indium and chlorine is indium (Ill) chloride, formula In Ciz. What electrons do you think indium gave up? Problem 15: When zinc combines with non-metals, it always has an oxidation number of +2. The electronic configuration of zinc is [Ar]3d1048", which is also written [Ar]4s 3d". What 2 electrons does zinc lose when it forms an ionic bond? Problem 16: When aluminum combines with non-metals, it always has an oxidation number of +3. What 3 electrons does aluminum lose when it forms an ionic bond? (See table above)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


