Question: Electrostatic interaction in the air or vacuum is a strong force, but is considered a weak molecular force in living systems (with water as the
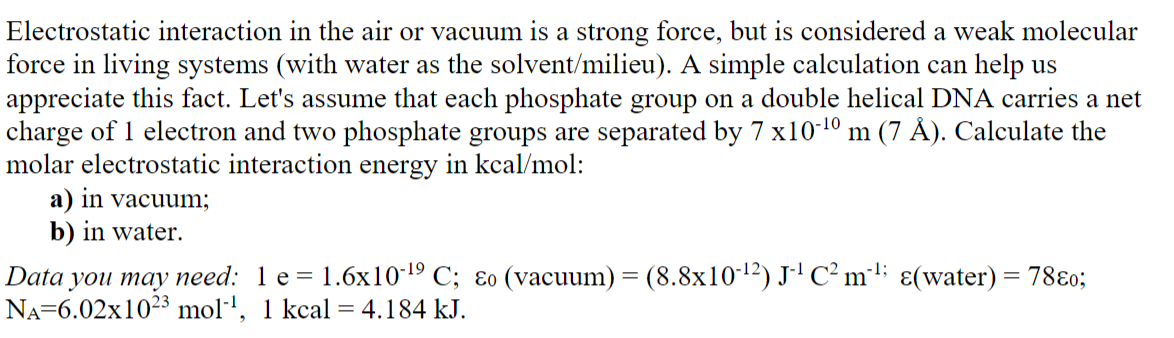
Electrostatic interaction in the air or vacuum is a strong force, but is considered a weak molecular force in living systems (with water as the solvent/milieu). A simple calculation can help us appreciate this fact. Let's assume that each phosphate group on a double helical DNA carries a net charge of 1 electron and two phosphate groups are separated by 7 x10-10 m (7 ). Calculate the molar electrostatic interaction energy in kcal/mol: a) in vacuum; b) in water. Data you may need: 1 e = 1.6x10-19 C; o (vacuum) = (8.8x10-12) J-4C m**; (water) = 78o; Na=6.02x1023 moll, 1 kcal = 4.184 kJ. -1; =
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


