Question: Emulsion is one of the problems we may face when performing extraction. The main reason that causes emulsion is O mixing the separatory funnel aggressively
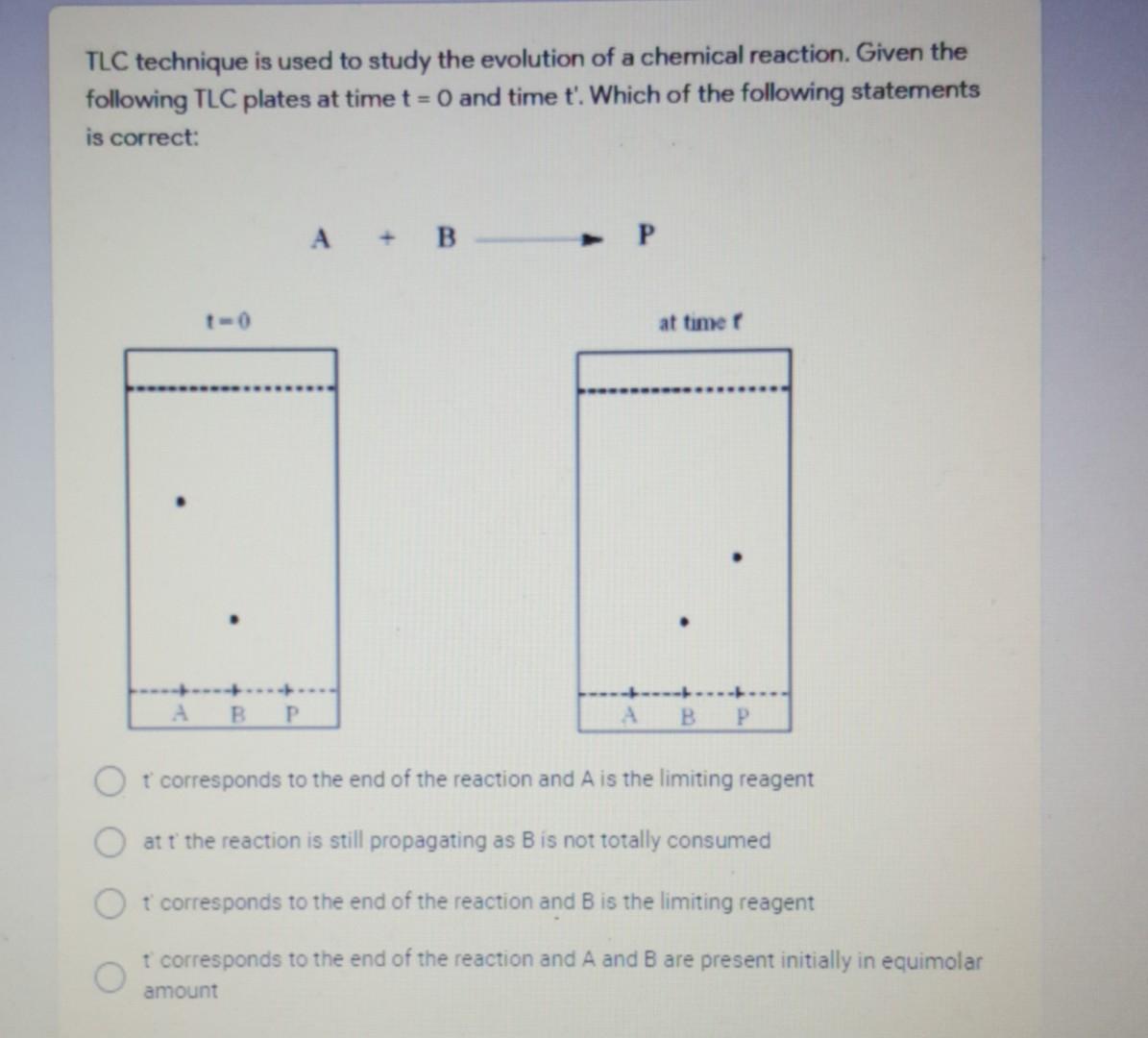

Emulsion is one of the problems we may face when performing extraction. The main reason that causes emulsion is O mixing the separatory funnel aggressively Useing a bigger separatory funnel O centrifugation Using a smaller separatory funnel a TLC technique is used to study the evolution of a chemical reaction. Given the following TLC plates at time t = 0 and time t'. Which of the following statements is correct: A + B-P at timer B B P t corresponds to the end of the reaction and A is the limiting reagent at t the reaction is still propagating as B is not totally consumed O t' corresponds to the end of the reaction and B is the limiting reagent t corresponds to the end of the reaction and A and B are present initially in equimolar amount The use of sodium carbonate solution during the ester work up will Hydrolyze the ester formed O react with any acid to produce CO2 gas O neutralize any acidity by forming H2 bubbling. Hydrolyze the excess of acetic acid
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


