Question: Enter electrons as e. Use smallest possible integer coefficients. States are not required. If a box is not needed, leave it blank. Standard Reduction
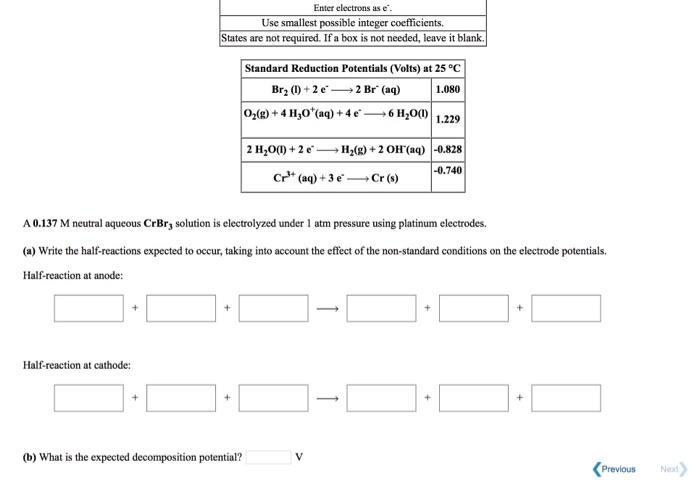

Enter electrons as e". Use smallest possible integer coefficients. States are not required. If a box is not needed, leave it blank. Standard Reduction Potentials (Volts) at 25 C Br, () + 2 e2 Br (aq) 1.080 0,(2) + 4 H,O*(aq) + 4e6 H,0) 1.229 2 H,O(1) + 2 e H2(g) + 2 OH (aq) -0.828 -0.740 Cr* (aq) + 3 eCr (9) A 0.137 M neutral aqueous CrBr, solution is electrolyzed under 1 atm pressure using platinum electrodes. (a) Write the half-reactions expected to occur, taking into account the effecet of the non-standard conditions on the electrode potentials. Half-reaction at anode: Half-reaction at cathode: (b) What is the expected decomposition potential? V Previous Nexd An aqueous MgF, solution is electrolyzed under 1 bar pressure using platinum electrodes. (a) Write the half-reactions predicted to occur at the anode and cathode, bascd on the standard cell potentials given below. Standard Reduction Potentials (Volts) at 25 C F,(2) + 2e2 F(aq) 2.870 0,(2) + 4 H,0*(aq) + 4e6 H,00 1.229 2 H,00) + 2e H(2) + 2 OH(aq) -0.828 Mg"(aq) + 2 eMg(9) -2.370 Half-reaction at anode: Half-reaction at cathode: (b) What is the expected decomposition potential? Enter electrons as e. Use smallest possible integer coefficients. States are not required. If a box is not needed, leave it blank.
Step by Step Solution
3.40 Rating (162 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


