Question: Enter your answer in the box provided. When entering your answer, enter the number only. Do not enter units. Suppose you have a 10.0 mL

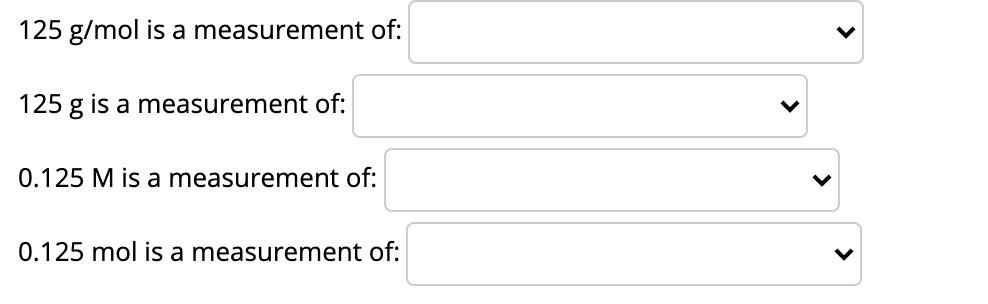
Enter your answer in the box provided. When entering your answer, enter the number only. Do not enter units. Suppose you have a 10.0 mL solution of the amino acid tyrosine with a concentration of 0.284 mg/mL. You take 2.00 mL of that solution and dilute it with 8.00 mL of water. You then add 4.00 mL of the diluted solution to an analysis tube. How many mg of tyrosine are in the analysis? 125 g/mol is a measurement of: 125 g is a measurement of: 0.125 M is a measurement of: 0.125 mol is a measurement of
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


