Question: Enter your answer in the provided box. A reaction vessel contains NH3, N2, and H, at equilibrium at a certain temperature. The equilibrium concentrations are
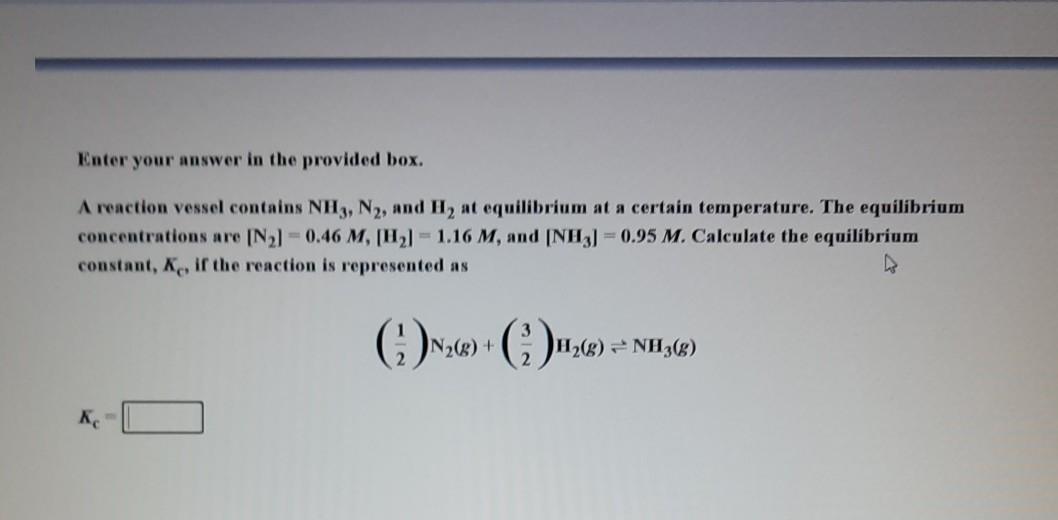
Enter your answer in the provided box. A reaction vessel contains NH3, N2, and H, at equilibrium at a certain temperature. The equilibrium concentrations are [2] -0.46 M, H21 - 1.16 M, and (NH3) = 0.95 M. Calculate the equilibrium constant, K, if the reaction is represented as N2(8) + H2(g) = NH3(8)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


