Question: Enter your answer in the provided box. A sample of sulfur hexafluoride gas occupies 7.79L at 206C. Assuming that the pressure remains constant, what temperature
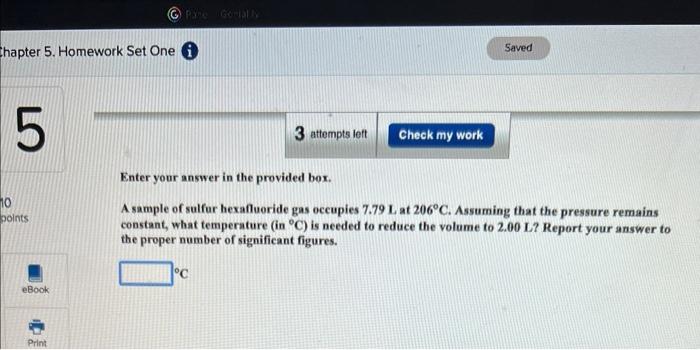
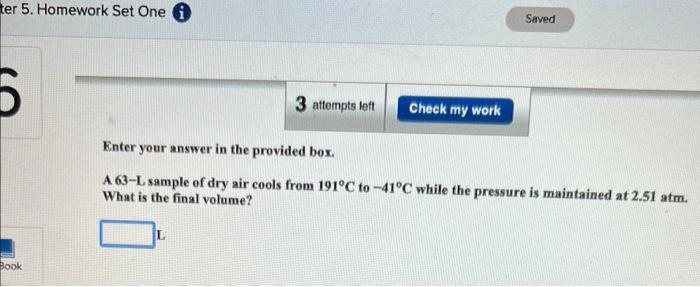
Enter your answer in the provided box. A sample of sulfur hexafluoride gas occupies 7.79L at 206C. Assuming that the pressure remains constant, what temperature (in C ) is needed to reduce the volume to 2.00L ? Report your answer to the proper number of significant figures. C Enter your answer in the provided box. A 63-L sample of dry air cools from 191C to 41C while the pressure is maintained at 2.51atm. What is the final volume? L
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


