Question: Enter your answer in the provided box. Element X has two naturally occurring isotopes, 65X (isotopic mass 64.7193 amu, abundance 72.47%) and 67x (isotopic mass
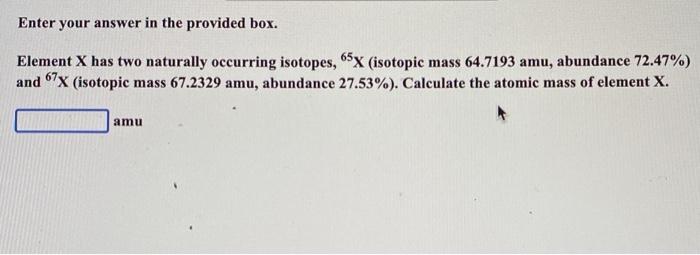
Enter your answer in the provided box. Element X has two naturally occurring isotopes, 65X (isotopic mass 64.7193 amu, abundance 72.47%) and 67x (isotopic mass 67.2329 amu, abundance 27.53%). Calculate the atomic mass of element X. amu
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


