Question: Enter your answer in the provided box. Even at high temperatures, the formation of NO is not favored: (Kc=4.10104at2000C) N2(g)+O2(g)2NO(g) What is [NO] when a
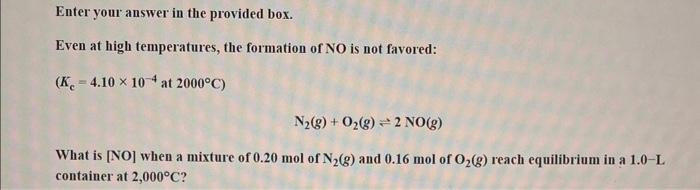
Enter your answer in the provided box. Even at high temperatures, the formation of NO is not favored: (Kc=4.10104at2000C) N2(g)+O2(g)2NO(g) What is [NO] when a mixture of 0.20mol of N2(g) and 0.16mol of O2(g) reach equilibrium in a 1.0-L container at 2,000C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


