Question: Enter your answer in the provided box. In an analysis of the following reaction at 100C, Br2(g) + Cl2(g) = 2BrCl(g) the equilibrium concentrations of
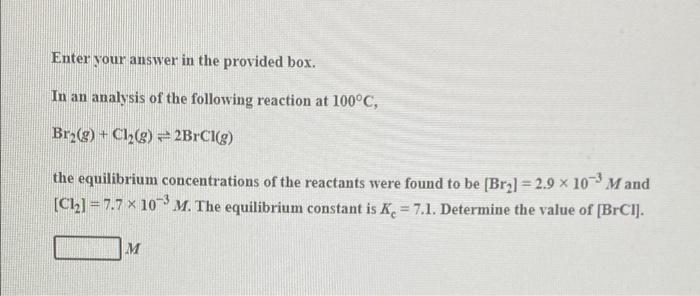
Enter your answer in the provided box. In an analysis of the following reaction at 100C, Br2(g) + Cl2(g) = 2BrCl(g) the equilibrium concentrations of the reactants were found to be [Bra] =2.9 x 10- Mand [C12] = 7.7 * 10-m. The equilibrium constant is K. = 7.1. Determine the value of (BrCi]. M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


