Question: Enter your answer in the provided box. Some commercial drain cleaners contain a mixture of sodium hydroxide and aluminum powder. When the mixture is poured
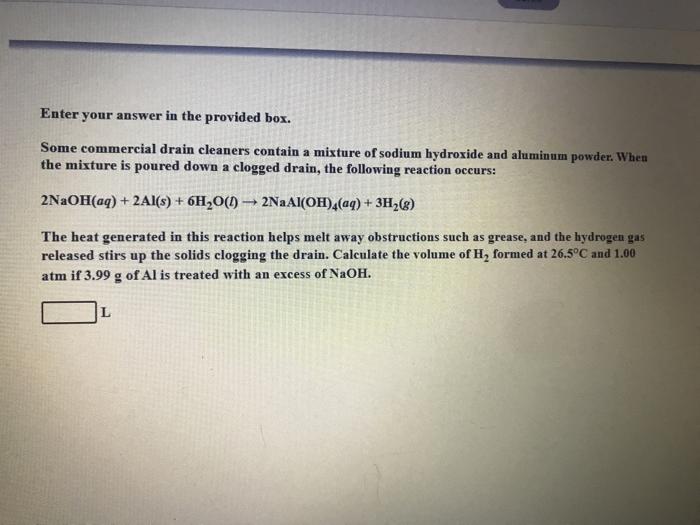
Enter your answer in the provided box. Some commercial drain cleaners contain a mixture of sodium hydroxide and aluminum powder. When the mixture is poured down a clogged drain, the following reaction occurs: 2NaOH(aq) + 2Al(s) + 6H20(1) - 2Na Al(OH)4(aq) + 3H2() The heat generated in this reaction helps melt away obstructions such as grease, and the hydrogen gas released stirs up the solids clogging the drain. Calculate the volume of H, formed at 26.5C and 1.00 atm if 3.99 g of Al is treated with an excess of NaOH. L
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


