Question: Enter your answer in the provided box. The rate constant for the second-order reaction 2NO2(g)2NO(g)+O2(g) is 0.54/M - s at 300C. How long (in seconds)
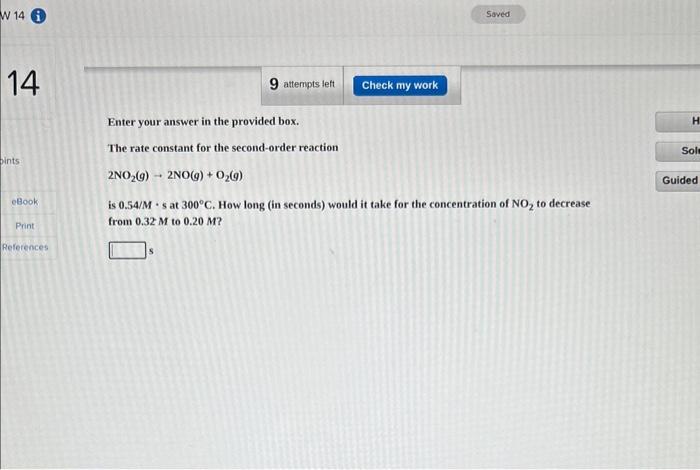
Enter your answer in the provided box. The rate constant for the second-order reaction 2NO2(g)2NO(g)+O2(g) is 0.54/M - s at 300C. How long (in seconds) would it take for the concentration of NO2 to decrease from 0.32M to 0.20M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


