Question: environmental engineering (4 pts) Two environmental processes that can release energy from biomass are aerobic respiration and denitrification: 41CH2O+41O241CO2+41H2O41CH2O+51NO3+51H+101N2+41CO2+207H2OGrespiration=29.9kcal/molGdenitrification=30.3kcal/mol According to the textbook, under typical
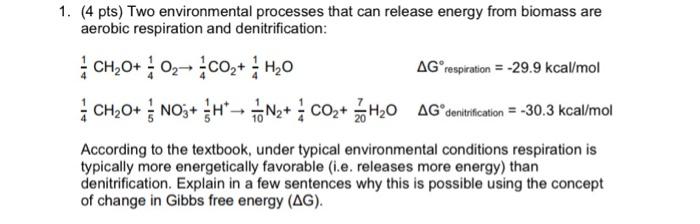
(4 pts) Two environmental processes that can release energy from biomass are aerobic respiration and denitrification: 41CH2O+41O241CO2+41H2O41CH2O+51NO3+51H+101N2+41CO2+207H2OGrespiration=29.9kcal/molGdenitrification=30.3kcal/mol According to the textbook, under typical environmental conditions respiration is typically more energetically favorable (i.e. releases more energy) than denitrification. Explain in a few sentences why this is possible using the concept of change in Gibbs free energy (G)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


