Question: -Equilibrium *NOTE: 1. please provide an answer for ALL the blanks 2. please notice the negative signs and the formulas used. last time someone answered
-Equilibrium *NOTE: 1. please provide an answer for ALL the blanks 2. please notice the negative signs and the formulas used. last time someone answered my question and missed the negative signs in the question which lead to incorrect answers. 3.please do not round the answers at all. write the full number with all the digits:) -please type the answers or write neatly:)... you can also fill in the blanks if you'd like to!
-please indicate part 1 and part 2.
PART1: TOTAL VOLUME IS: 15ml
PART 2:
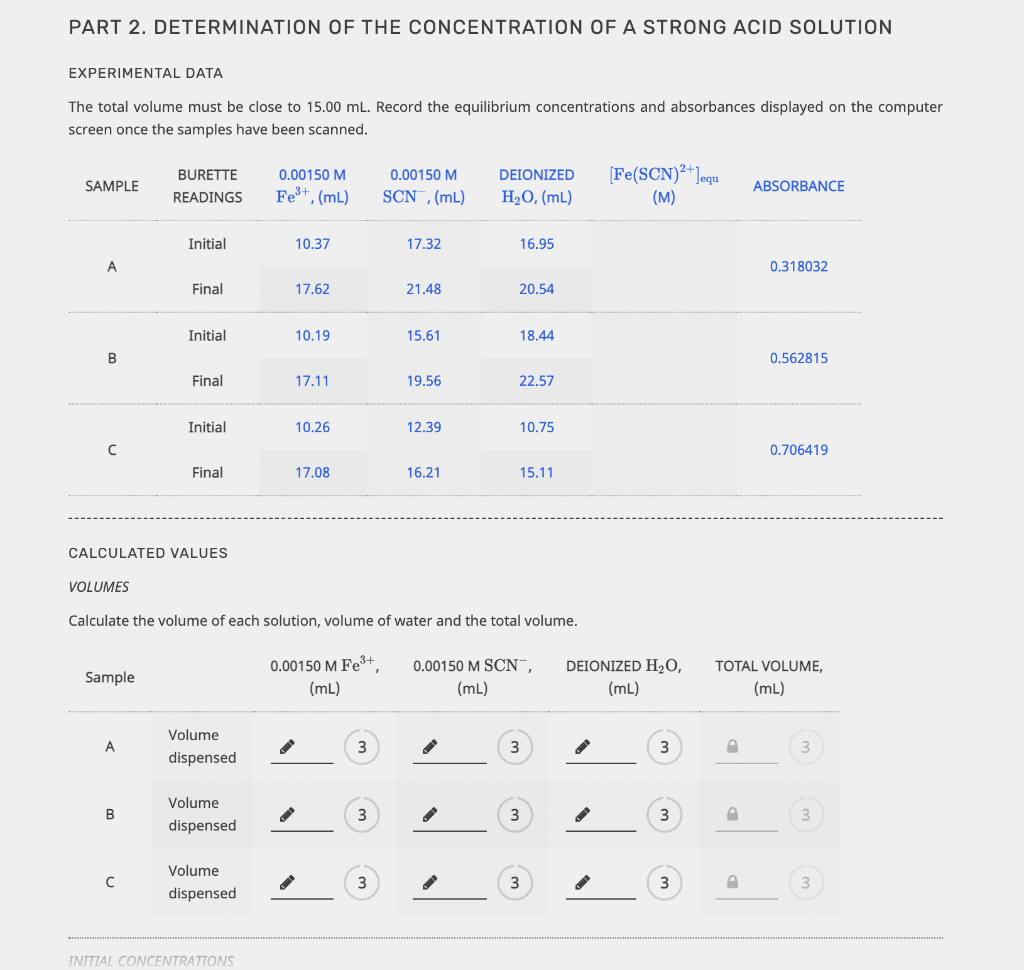

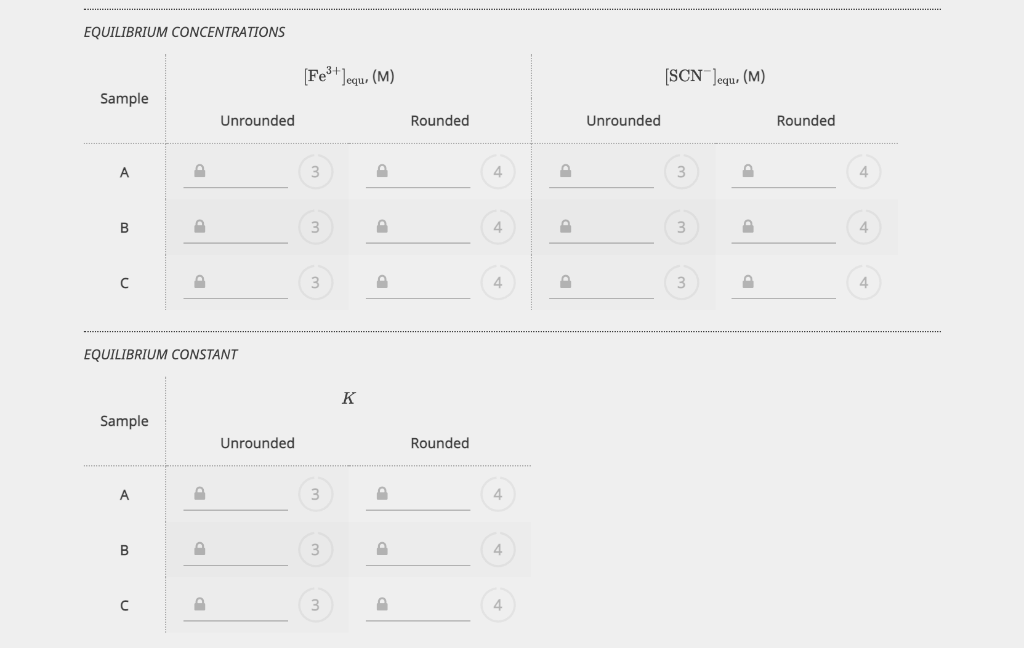
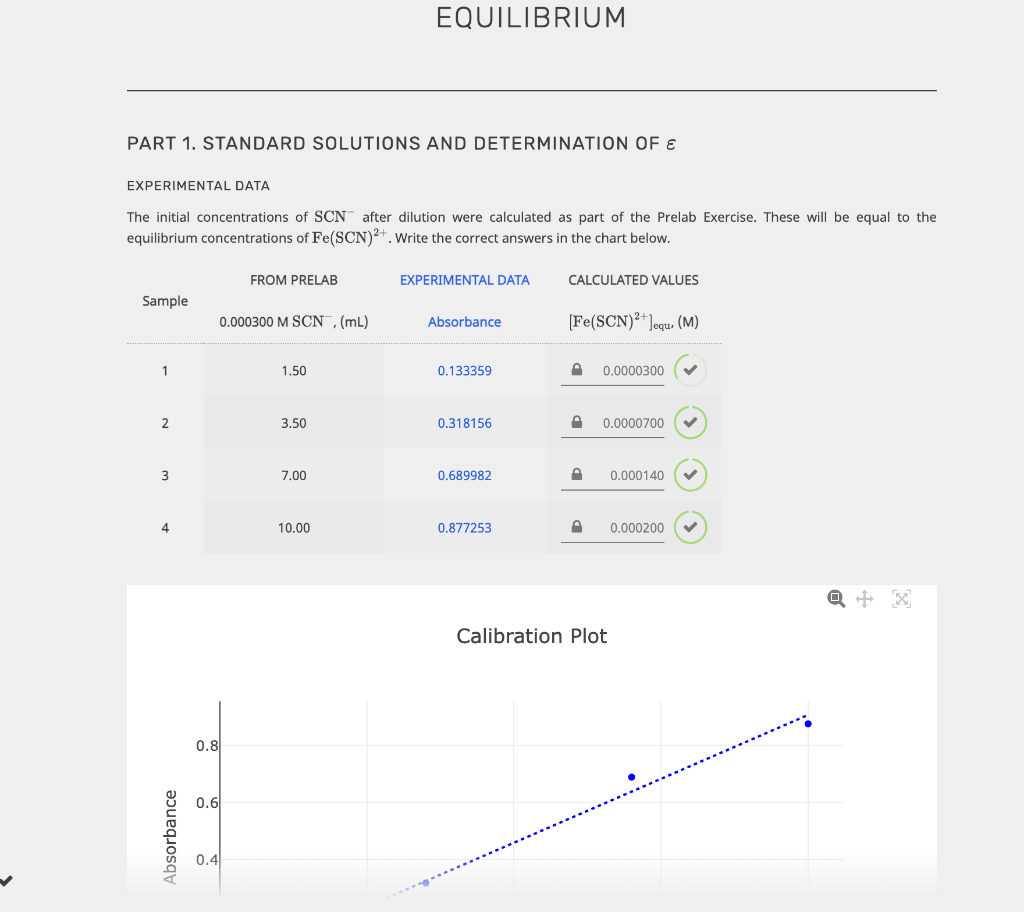
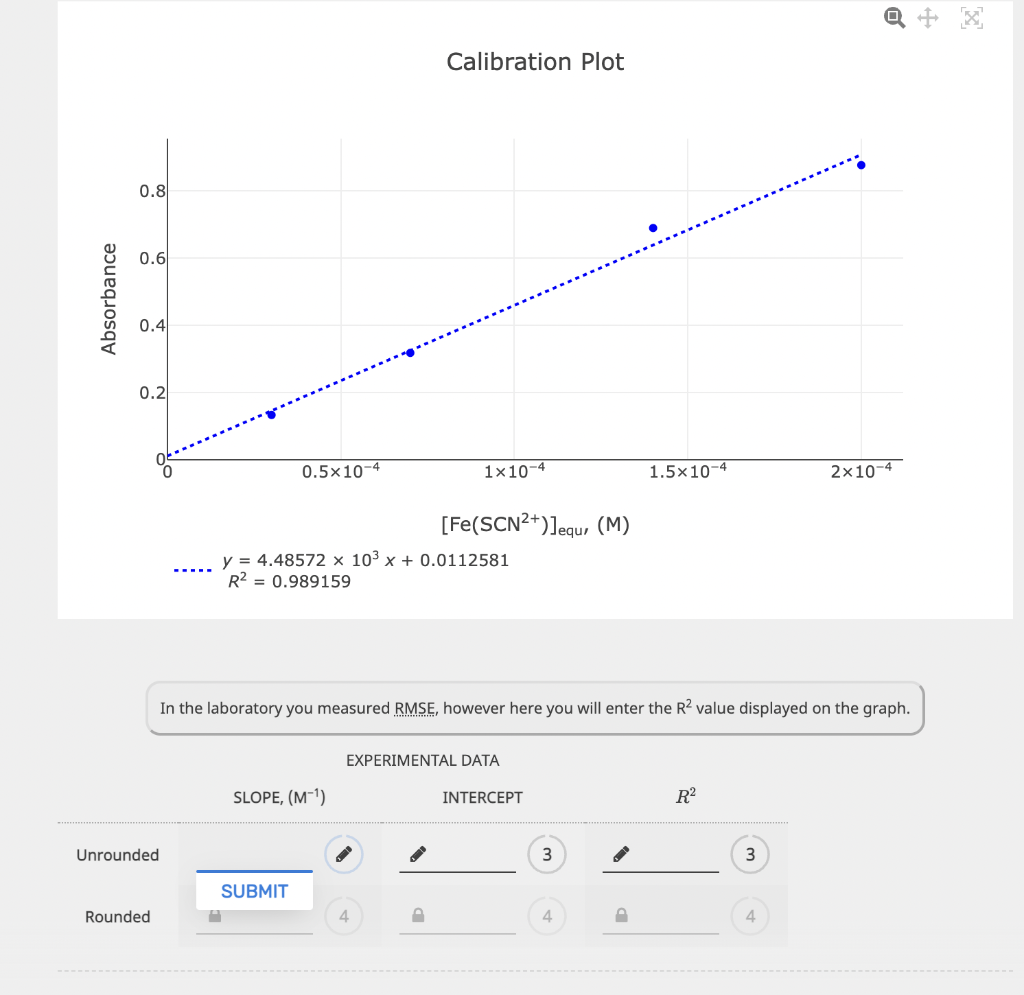
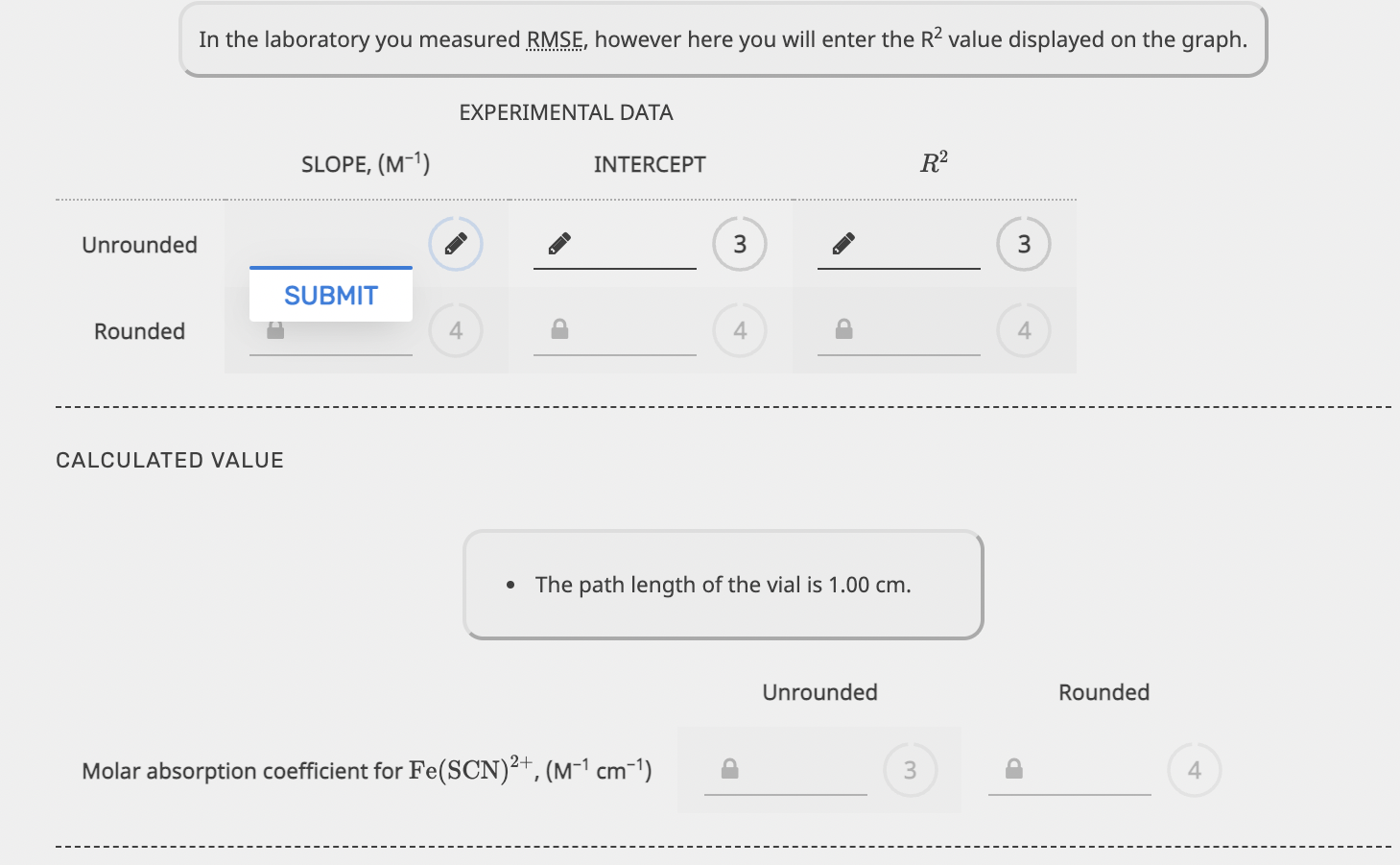
EQUILIBRIUM PART 1. STANDARD SOLUTIONS AND DETERMINATION OF E EXPERIMENTAL DATA The initial concentrations of SCN after dilution were calculated as part of the Prelab Exercise. These will be equal to the equilibrium concentrations of Fe(SCN)2+. Write the correct answers in the chart below. FROM PRELAB EXPERIMENTAL DATA CALCULATED VALUES Sample 0.000300 M SCN ,(mL) Absorbance [Fe(SCN)2+]equ. (M) 1 1.50 0.133359 0.0000300 2 3.50 0.318156 0.0000700 3 7.00 0.689982 0.000140 4 10.00 0.877253 0.000200 Calibration Plot 0.8 0.6 Absorbance 0.4 Calibration Plot 0.8 0.6 Absorbance 0.4 0.2 0.5x 10-4 1x10-4 1.5x10-4 2x10-4 [Fe(SCN2+)]equ, (M) y = 4.48572 x 103 x + 0.0112581 R2 = 0.989159 In the laboratory you measured RMSE, however here you will enter the R2 value displayed on the graph. EXPERIMENTAL DATA SLOPE, (M-1) INTERCEPT R2 Unrounded 3 3 SUBMIT Rounded 4 In the laboratory you measured RMSE, however here you will enter the R2 value displayed on the graph. EXPERIMENTAL DATA SLOPE, (M-1). INTERCEPT R2 Unrounded 3 3 SUBMIT Rounded 4 4 4 CALCULATED VALUE The path length of the vial is 1.00 cm. Unrounded Rounded Molar absorption coefficient for Fe(SCN)2+, (M-1 cm+1) 3 4 PART 2. DETERMINATION OF THE CONCENTRATION OF A STRONG ACID SOLUTION EXPERIMENTAL DATA The total volume must be close to 15.00 ml. Record the equilibrium concentrations and absorbances displayed on the computer screen once the samples have been scanned. 0.00150 M SAMPLE BURETTE READINGS 0.00150 M SCN,(mL) DEIONIZED H,0, (mL) Fe(SCN)2+Jequ (M) Fe3+,(mL) ABSORBANCE Initial 10.37 17.32 16.95 0.318032 Final 17.62 21.48 20.54 Initial 10.19 15.61 18.44 B 0.562815 Final 17.11 19.56 22.57 Initial 10.26 12.39 10.75 0.706419 Final 17.08 16.21 15.11 CALCULATED VALUES VOLUMES Calculate the volume of each solution, volume of water and the total volume. Sample 0.00150 M Fe3+ (mL) 0.00150 M SCN, (mL) DEIONIZED H2O (mL) TOTAL VOLUME, (mL) A Volume dispensed 3 3 B Volume dispensed 3 3 3 Volume dispensed . 3 3 3 3 INITIAL CONCENTRATIONS INITIAL CONCENTRATIONS Now determine the (FeS+Jinitial and (SCN)initial after mixing but before the reaction occurs. Fe3+ initial, (M) [SCN Jinitial, (M) Sample Unrounded Rounded Unrounded Rounded A B 3 4 P 3 D 3 D 3 D EQUILIBRIUM CONCENTRATIONS [Fe3+) Jequ. (M) [SCN]equ. (M) Sample Unrounded Rounded Unrounded Rounded A 3 4 3 B 3 4 3 4 D 3 4 P 3 D 4 EQUILIBRIUM CONSTANT K Sample Unrounded Rounded A 3 B 3 4 EQUILIBRIUM CONCENTRATIONS Fe3+ Jequ. (M) [SCN]equ. (M) Sample Unrounded Rounded Unrounded Rounded 3 D 4 P 3 P 4 B D 3 4 3 4 3 P 4 P 3 D 4 EQUILIBRIUM CONSTANT K Sample Unrounded Rounded B 3 D
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


