Question: Equilibrium Type your answer to Part (a), Part (b) and Part (c) of this question in the box below. The following question refers to the
Equilibrium
Type your answer to Part (a), Part (b) and Part (c) of this question in the box below.
The following question refers to the equilibrium described by:
2NOBr(g) 2NO(g) + Br2(g)
The top graph below shows the change in equilibrium concentration of NOBr when more NOBr is added to the reaction.
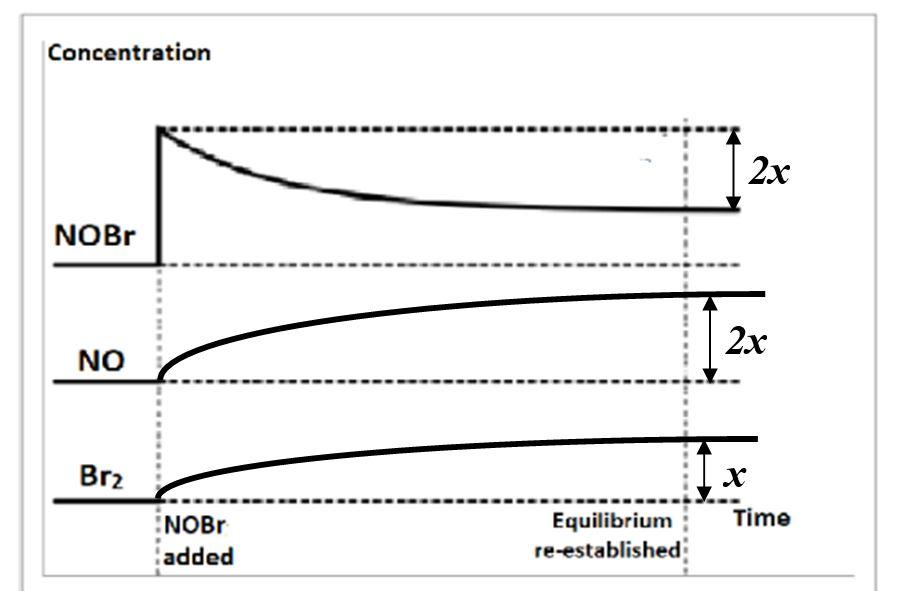
(a) The corresponding curves for NO and Br2 have been drawn, showing the relative changes in their concentrations in terms of x. Explain these changes. (2 marks)
(b) The following question refers to the equilibrium described by:
2NOBr(g) 2NO(g) + Br2(g) H > 0
The reaction as written is endothermic.
After the system was allowed to reach equilibrium, the temperature was decreased.
What would happen to the concentration of concentration of Br2 as a result of this change? Explain your answer, making reference to the equilibrium. (2 marks)
(c) The following question refers to the equilibrium described by:
2NOBr(g) 2NO(g) + Br2(g)
After the system was allowed to reach equilibrium, the volume of the reaction container was decreased.
What would happen to the concentration of concentration of NOBr as a result of this change? Explain your answer, making reference to the equilibrium.
PLEASE BEIRFLY AND TYPE THE ANSWER (DON'T HAND WRITE IT)
Concentration 2x NOBr 2x NO Br2 X Time NOBO added Equilibrium re-established
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


