Question: ered SHOW ALL YOUR WORK. Write the answer in the following box and upload your solutions to the Part 2 (if you cannot complete you
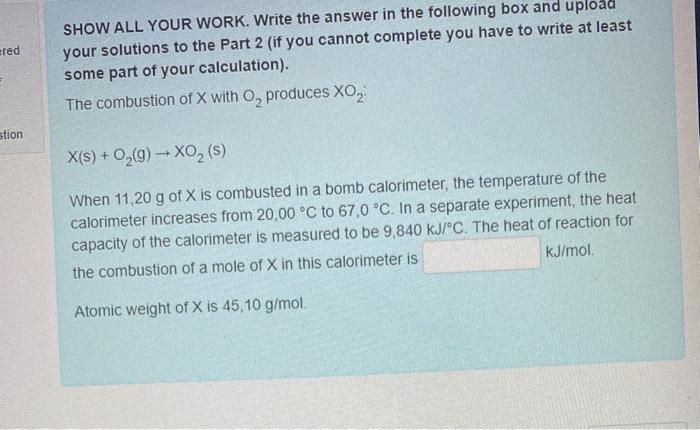
ered SHOW ALL YOUR WORK. Write the answer in the following box and upload your solutions to the Part 2 (if you cannot complete you have to write at least some part of your calculation). The combustion of X with O, produces XO, stion X(s) + O2(9) - XO2 (s) When 11,20 g of X is combusted in a bomb calorimeter, the temperature of the calorimeter increases from 20,00 C to 67,0 C. In a separate experiment, the heat capacity of the calorimeter is measured to be 9,840 kJ/C. The heat of reaction for the combustion of a mole of X in this calorimeter is kJ/mol Atomic weight of X is 45,10 g/mol
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


