Question: Ethanol fermentation -- simplified An alcoholic fermentation process produces 20 kg of product that is 6% by weight ethanol. Assume the balance is water. The
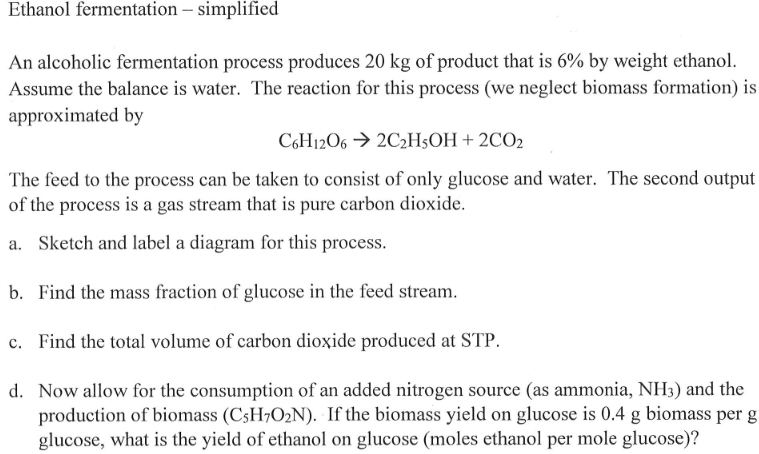
Ethanol fermentation -- simplified An alcoholic fermentation process produces 20 kg of product that is 6% by weight ethanol. Assume the balance is water. The reaction for this process (we neglect biomass formation) is approximated by C6H1206 2C2H5OH + 2CO2 The feed to the process can be taken to consist of only glucose and water. The second output of the process is a gas stream that is pure carbon dioxide. a. Sketch and label a diagram for this process. b. Find the mass fraction of glucose in the feed stream. c. Find the total volume of carbon dioxide produced at STP. d. Now allow for the consumption of an added nitrogen source (as ammonia, NH3) and the production of biomass (C3H7O2N). If the biomass yield on glucose is 0.4 g biomass per g glucose, what is the yield of ethanol on glucose (moles ethanol per mole glucose)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


