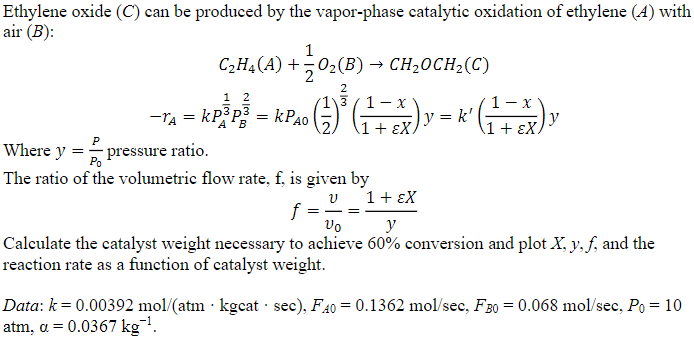
Question: Ethylene oxide ( C ) can be produced by the vapor - phase catalytic oxidation of ethylene ( A ) with air ( B )
Ethylene oxide can be produced by the vaporphase catalytic oxidation of ethylene with
air :
Where pressure ratio.
The ratio of the volumetric flow rate, is given by
Calculate the catalyst weight necessary to achieve conversion and plot and the
reaction rate as a function of catalyst weight.
Data:
atm,
Hint: Use bisection method and the builtin ode solver in PYTHON.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


