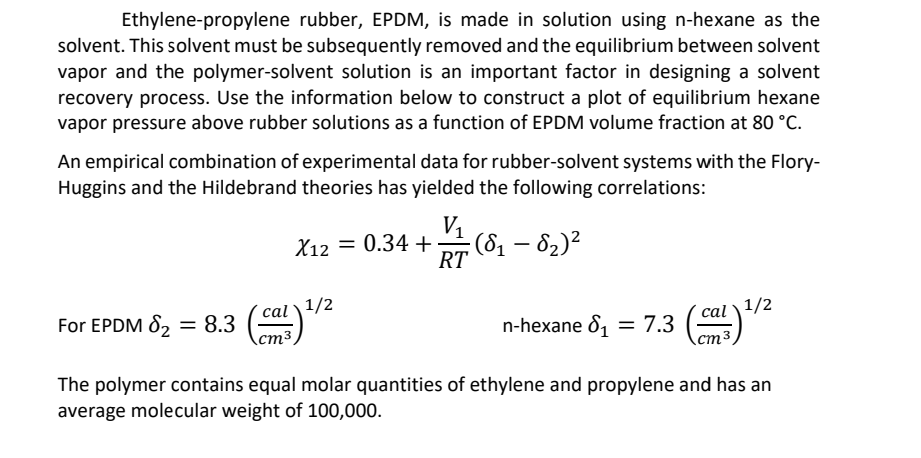
Question: Ethylene - propylene rubber, EPDM, is made in solution using n - hexane as the solvent. This solvent must be subsequently removed and the equilibrium
Ethylenepropylene rubber, EPDM, is made in solution using hexane as the solvent. This solvent must be subsequently removed and the equilibrium between solvent vapor and the polymersolvent solution is an important factor in designing a solvent recovery process. Use the information below to construct a plot of equilibrium hexane vapor pressure above rubber solutions as a function of EPDM volume fraction at
An empirical combination of experimental data for rubbersolvent systems with the FloryHuggins and the Hildebrand theories has yielded the following correlations:
For EPDM nhexane
The polymer contains equal molar quantities of ethylene and propylene and has an average molecular weight of
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


