Question: Eutectic tin-lead solder (Tin 63% / Lead 37% ) was the most popular type of solder used in electronic devices for many years. The below
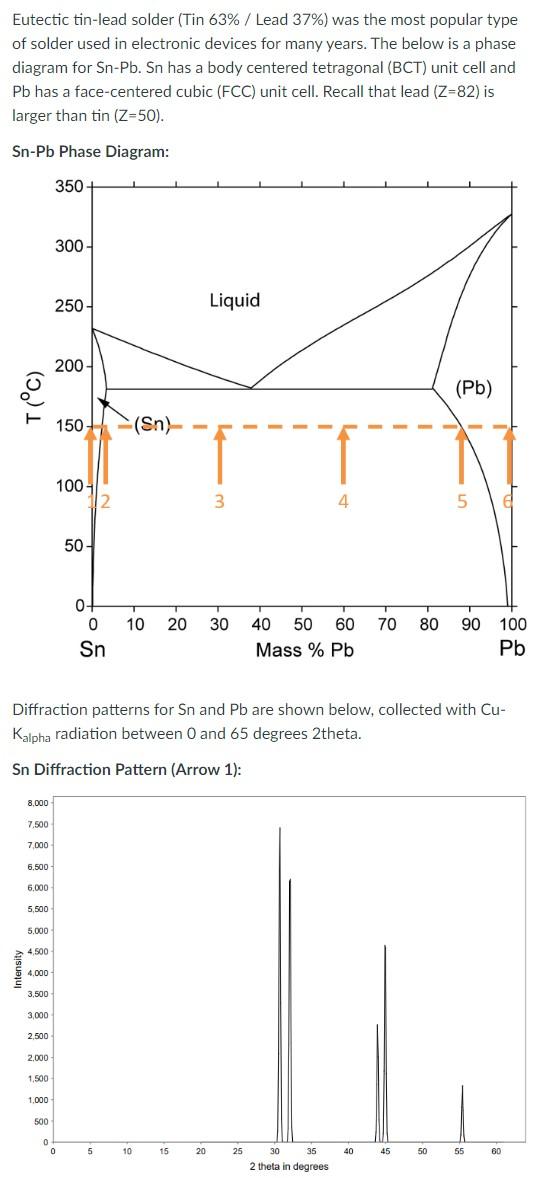
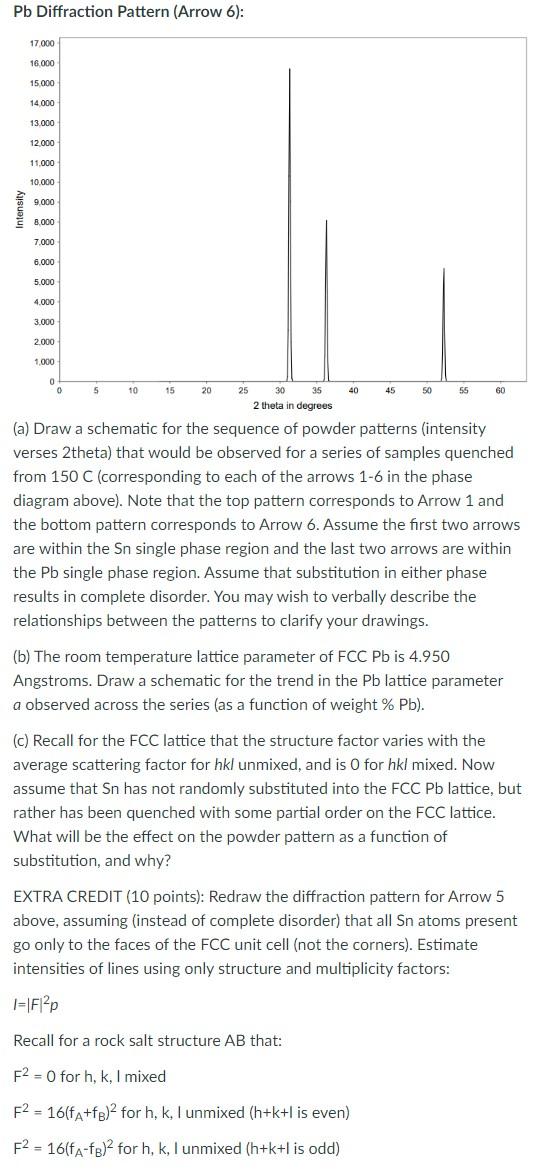
Eutectic tin-lead solder (Tin 63% / Lead 37% ) was the most popular type of solder used in electronic devices for many years. The below is a phase diagram for Sn-Pb. Sn has a body centered tetragonal (BCT) unit cell and Pb has a face-centered cubic (FCC) unit cell. Recall that lead (Z=82) is larger than tin (Z=50). Sn-Pb Phase Diagram: Diffraction patterns for Sn and Pb are shown below, collected with Cu Kalpha radiation between 0 and 65 degrees 2theta. Sn Diffraction Pattern (Arrow 1): Pb Diffraction Pattern (Arrow 6): (a) Draw a schematic for the sequence of powder patterns (intensity verses 2 theta) that would be observed for a series of samples quenched from 150C (corresponding to each of the arrows 1-6 in the phase diagram above). Note that the top pattern corresponds to Arrow 1 and the bottom pattern corresponds to Arrow 6. Assume the first two arrows are within the Sn single phase region and the last two arrows are within the Pb single phase region. Assume that substitution in either phase results in complete disorder. You may wish to verbally describe the relationships between the patterns to clarify your drawings. (b) The room temperature lattice parameter of FCCPb is 4.950 Angstroms. Draw a schematic for the trend in the Pb lattice parameter a observed across the series (as a function of weight \% Pb ). (c) Recall for the FCC lattice that the structure factor varies with the average scattering factor for hkl unmixed, and is 0 for hkl mixed. Now assume that Sn has not randomly substituted into the FCC Pb lattice, but rather has been quenched with some partial order on the FCC lattice. What will be the effect on the powder pattern as a function of substitution, and why? EXTRA CREDIT (10 points): Redraw the diffraction pattern for Arrow 5 above, assuming (instead of complete disorder) that all Sn atoms present go only to the faces of the FCC unit cell (not the corners). Estimate intensities of lines using only structure and multiplicity factors: I=F2p Recall for a rock salt structure AB that: F2=0 for h,k,I mixed F2=16(fA+fB)2 for h,k, unmixed (h+k+l is even ) F2=16(fAfB)2 for h,k, unmixed (h+k+l is odd) Eutectic tin-lead solder (Tin 63% / Lead 37% ) was the most popular type of solder used in electronic devices for many years. The below is a phase diagram for Sn-Pb. Sn has a body centered tetragonal (BCT) unit cell and Pb has a face-centered cubic (FCC) unit cell. Recall that lead (Z=82) is larger than tin (Z=50). Sn-Pb Phase Diagram: Diffraction patterns for Sn and Pb are shown below, collected with Cu Kalpha radiation between 0 and 65 degrees 2theta. Sn Diffraction Pattern (Arrow 1): Pb Diffraction Pattern (Arrow 6): (a) Draw a schematic for the sequence of powder patterns (intensity verses 2 theta) that would be observed for a series of samples quenched from 150C (corresponding to each of the arrows 1-6 in the phase diagram above). Note that the top pattern corresponds to Arrow 1 and the bottom pattern corresponds to Arrow 6. Assume the first two arrows are within the Sn single phase region and the last two arrows are within the Pb single phase region. Assume that substitution in either phase results in complete disorder. You may wish to verbally describe the relationships between the patterns to clarify your drawings. (b) The room temperature lattice parameter of FCCPb is 4.950 Angstroms. Draw a schematic for the trend in the Pb lattice parameter a observed across the series (as a function of weight \% Pb ). (c) Recall for the FCC lattice that the structure factor varies with the average scattering factor for hkl unmixed, and is 0 for hkl mixed. Now assume that Sn has not randomly substituted into the FCC Pb lattice, but rather has been quenched with some partial order on the FCC lattice. What will be the effect on the powder pattern as a function of substitution, and why? EXTRA CREDIT (10 points): Redraw the diffraction pattern for Arrow 5 above, assuming (instead of complete disorder) that all Sn atoms present go only to the faces of the FCC unit cell (not the corners). Estimate intensities of lines using only structure and multiplicity factors: I=F2p Recall for a rock salt structure AB that: F2=0 for h,k,I mixed F2=16(fA+fB)2 for h,k, unmixed (h+k+l is even ) F2=16(fAfB)2 for h,k, unmixed (h+k+l is odd)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


