Question: Evalua- 1 pts wer Question 5 atrix a ams . Which of the following chemical formulas are empirical formulas? Select all that apply. Hg2(NO3)2 urces
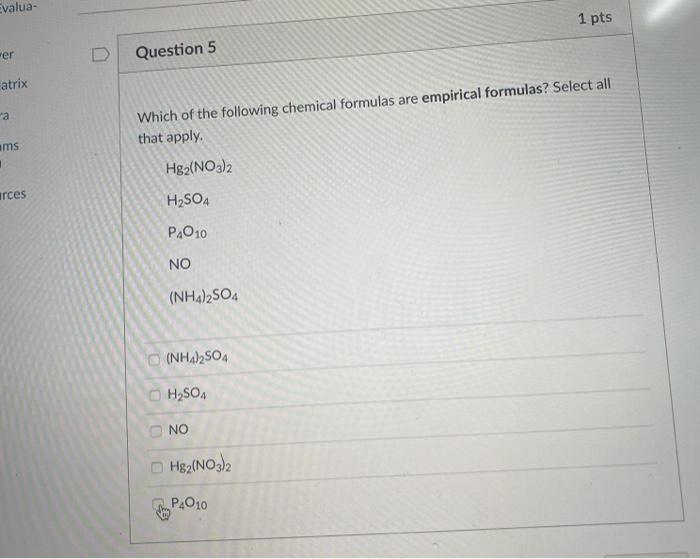
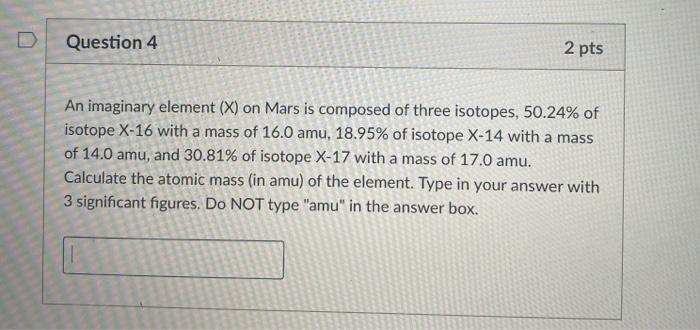
Evalua- 1 pts wer Question 5 atrix a ams . Which of the following chemical formulas are empirical formulas? Select all that apply. Hg2(NO3)2 urces H2SO4 PAO10 NO (NH4)2SO4 C (NH4)2SO4 H2SO4 NO H82(NO3)2 P4010 Question 4 2 pts An imaginary element (X) on Mars is composed of three isotopes, 50.24% of isotope X-16 with a mass of 16. amu, 18.95% of isotope X-14 with a mass of 14.0 amu, and 30.81% of isotope X-17 with a mass of 17.0 amu. Calculate the atomic mass (in amu) of the element. Type in your answer with 3 significant figures. Do NOT type "amu" in the answer box
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


