Question: Example 16.12 Practice Problems 1. Given ly = 1.6 s Required time required for 99% of astatine in a sample to decay (t) Analysis and
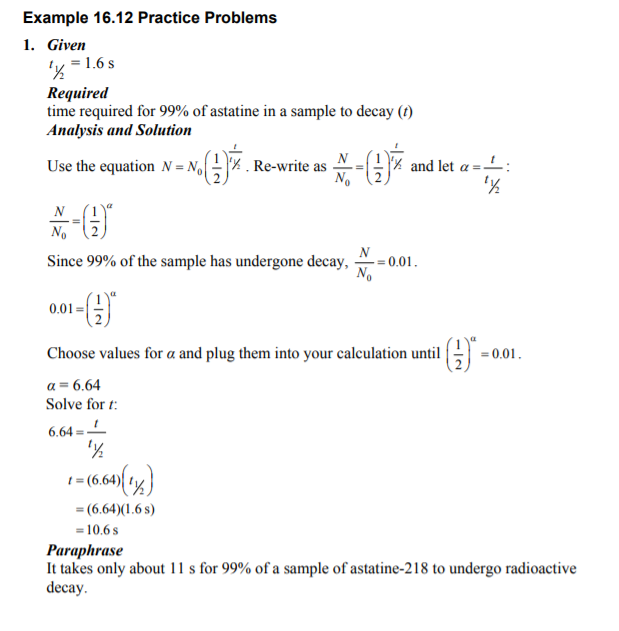
Example 16.12 Practice Problems 1. Given ly = 1.6 s Required time required for 99% of astatine in a sample to decay (t) Analysis and Solution Use the equation N = N. _ 'x . Re-write as 6% and let a = 1: N No Since 99% of the sample has undergone decay, -=0.01. 0.01 = Choose values for a and plug them into your calculation until NI- = 0.01. a = 6.64 Solve for t: 6.64 =4 1 = (6.64)(14) = (6.64)(1.6s) =10.6s Paraphrase It takes only about 1 1 s for 99% of a sample of astatine-218 to undergo radioactive decay
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


