Question: Example 30. While determining the pH of solation, the quinbydrone electrode, #*, Q. QHz was used in conjunction with a saturated calomel electrode, as represented
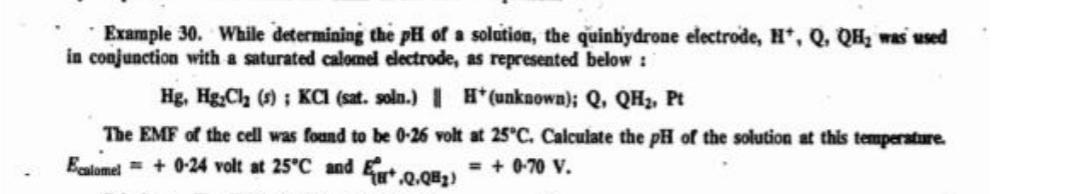
Example 30. While determining the pH of solation, the quinbydrone electrode, #*, Q. QHz was used in conjunction with a saturated calomel electrode, as represented below : Hg, HgCl2 () ; KCI (sat. soln.) 1 * (unknown); Q, QH2, Pt The EMF of the cell was found to be 0-26 volt at 25C. Calculate the pH of the solution at this temperature Ecatomel = + 0-24 volt at 25C and Ex+.2.Q8z) = + 0-70 v
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


