Question: Example - 7: The Winkler method uses redox reactions to find the concentration of oxygen in water. 100cm3 of water was taken from a river
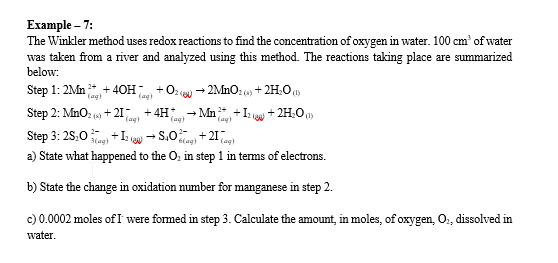
Example - 7: The Winkler method uses redox reactions to find the concentration of oxygen in water. 100cm3 of water was taken from a river and analyzed using this method. The reactions taking place are summarized below: Step 1: 2Mn(aqg)2++4OH(aqg)+O2(ev)2MnO2(s)+2H2O(1) Step 2: MnO2(s)+2I(aqg)+4H(aqg)+Mn(aqq)2++I2(aj)+2H2O(l) Step 3: 2S2O3(aq)2+I2(aj)S4O6(aq)2+2I(aqg) a) State what happened to the O2 in step 1 in terms of electrons. b) State the change in oxidation number for manganese in step 2. c) 0.0002 moles of I were formed in step 3. Calculate the amount, in moles, of oxygen, O2, dissolved in water
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


