Question: Example Calculation 1.1.1 Use equations (1.1.1 and 1.12 ) to determine the (1) absolute error and (2) percent error for a value of 4.422g/cm3 determined
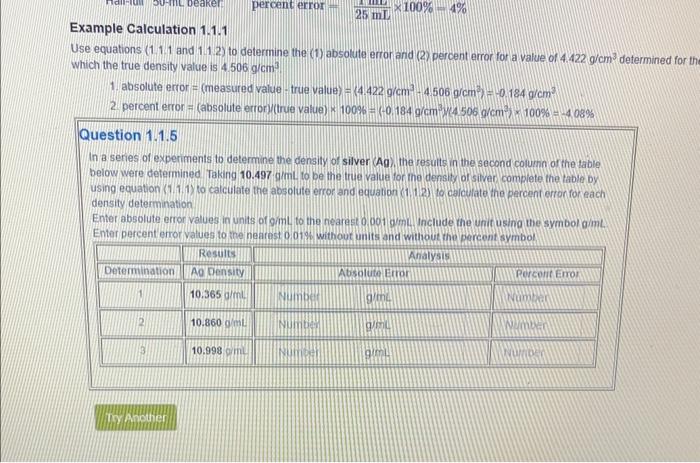
Example Calculation 1.1.1 Use equations (1.1.1 and 1.12 ) to determine the (1) absolute error and (2) percent error for a value of 4.422g/cm3 determined for th Which the true density value is 4.506gcm3 1. absolute error =( measured value true value )={4.422g/cm34.5069(cm3)=0.184g/cm3)2 2 percent error = (absolute error) ( true value) 100%=(0.184gcm3)(4.506.9/cm3)100%=4.03% Question 1.1.5 In a senies of experiments to determine the density of silver (Ag), the results in the second columin of the table? below were determined Taking 10.497 gimL to be the true value for me densitto or siven complete the table by using equation (11.1.1) to calculate the absolute error and equation (1.12) to calculate the percent erronforeach density determination Enter absolute error values in units of gimL to the nearest on oot omm. Include fhe unit using the symbol aimL. Enter percent errot values to the neatest o. 01s without units and without the bercent svmbol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


