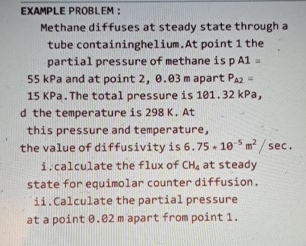
Question: EXAMPLE PROBLEM : Methane diffuses at steady state through a tube containing helium. At point 1 the partial pressure of methane is p A 1
EXAMPLE PROBLEM :
Methane diffuses at steady state through a tube containing helium. At point the partial pressure of methane is kPa and at point apart KPa. The total pressure is kPa, d the temperature is At this pressure and temperature, the value of diffusivity is
i calculate the flux of at steady state for equimolar counter diffusion.
ii Calculate the partial pressure at a point apart from point
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


