Question: Example: Using Hess's law determine the enthalpy of formation, H, of FeCl3 (s) from the enthalpy changes of the following two step process that occurs
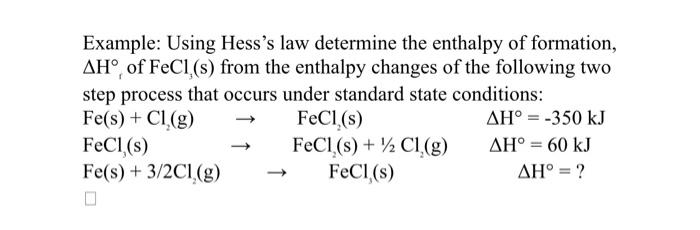
Example: Using Hess's law determine the enthalpy of formation, H, of FeCl3 (s) from the enthalpy changes of the following two step process that occurs under standard state conditions: Fe(s)+Cl2(g)FeCl2(s)Fe(s)+3/2Cl2(g)FeCl2(s)FeCl2(s)+1/2Cl2(g)FeCl3(s)H=350kJH=60kJH=
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


