Question: Exercise 1 0 . Sucrose ( C 1 2 H 2 2 O 1 1 ) undergoes hydrolysis to form fructose ( C 6 H
Exercise Sucrose undergoes hydrolysis to form fructose and glucose :
This reaction is important in the candy industry. First, fructose is sweeter than sucrose. In addition, a mixture of fructose and glucose will not crystallize. This makes the sweets elastic.
a Based on the following data, determine the order of the reaction
b How long does it take to hydrolyse sucrose?
min
first order
c Explain why the speed equation does not contain while water is a reagent.
water does no make sense to hue a concontration
Is doen't detect the rute
tableTime min
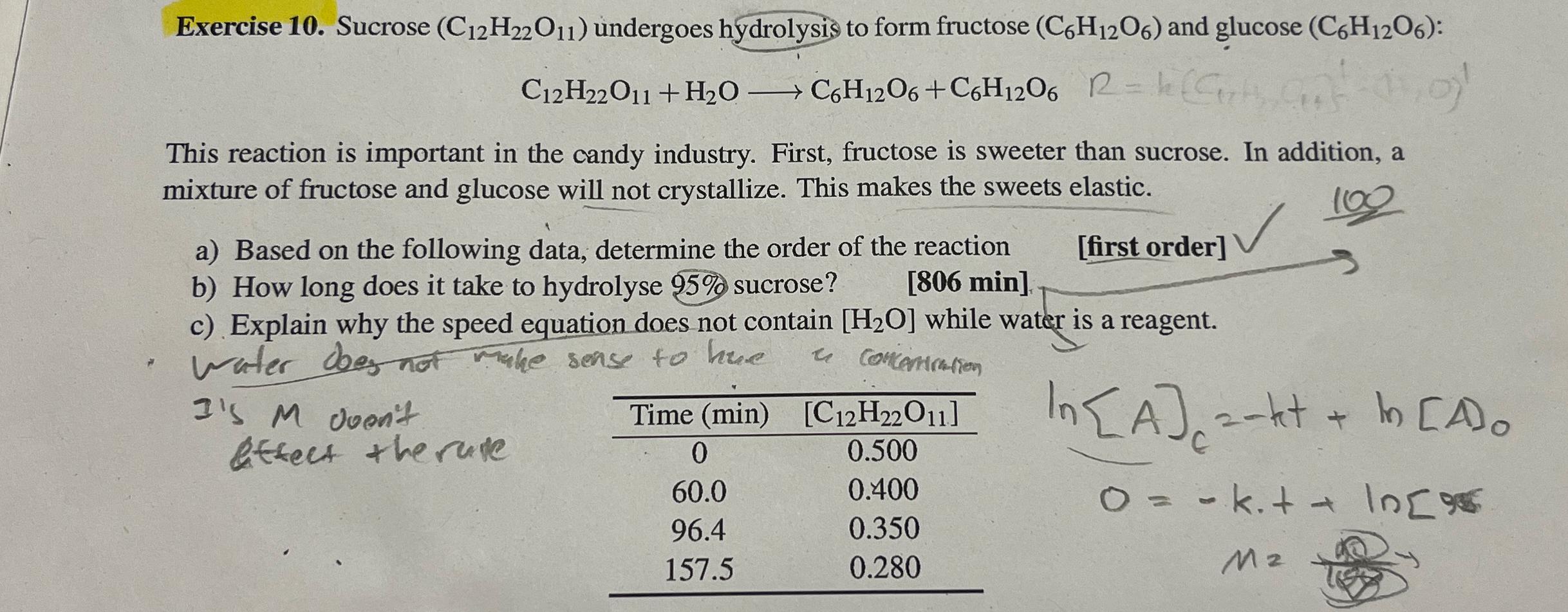
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


