Question: Exercise: 1 (5 Marks) Calculate the diffusivity in m2/s of carbon in HCP titanium at 700C. Use D0=5.10104m2/s;Q=182kJ/mol;R=8.314J/(molK). Exercise: 2 (2 Marks) Nickel, Aluminum &
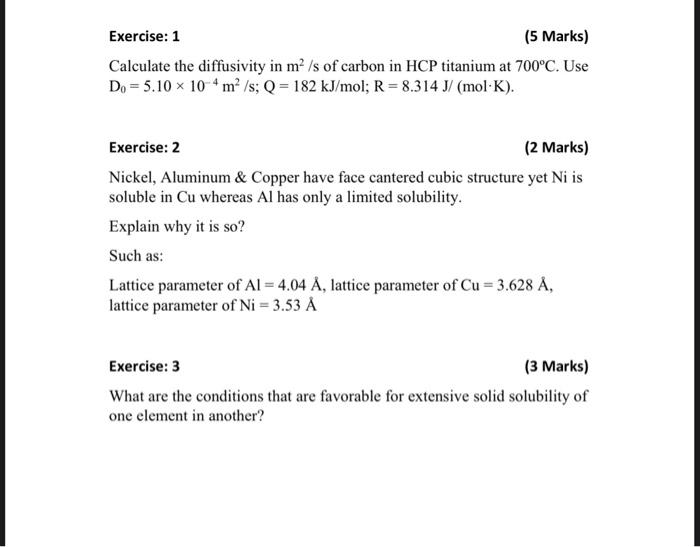
Exercise: 1 (5 Marks) Calculate the diffusivity in m2/s of carbon in HCP titanium at 700C. Use D0=5.10104m2/s;Q=182kJ/mol;R=8.314J/(molK). Exercise: 2 (2 Marks) Nickel, Aluminum \& Copper have face cantered cubic structure yet Ni is soluble in Cu whereas Al has only a limited solubility. Explain why it is so? Such as: Lattice parameter of Al=4.04A, lattice parameter of Cu=3.628A, lattice parameter of Ni=3.53A Exercise: 3 (3 Marks) What are the conditions that are favorable for extensive solid solubility of one element in another
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


