Question: Exercise 1: The ternary phase diagram below shows the phase behaviour for the 3-component system of water, anionic surfactant NaC8 and C10OH (co-surfactant). a. What
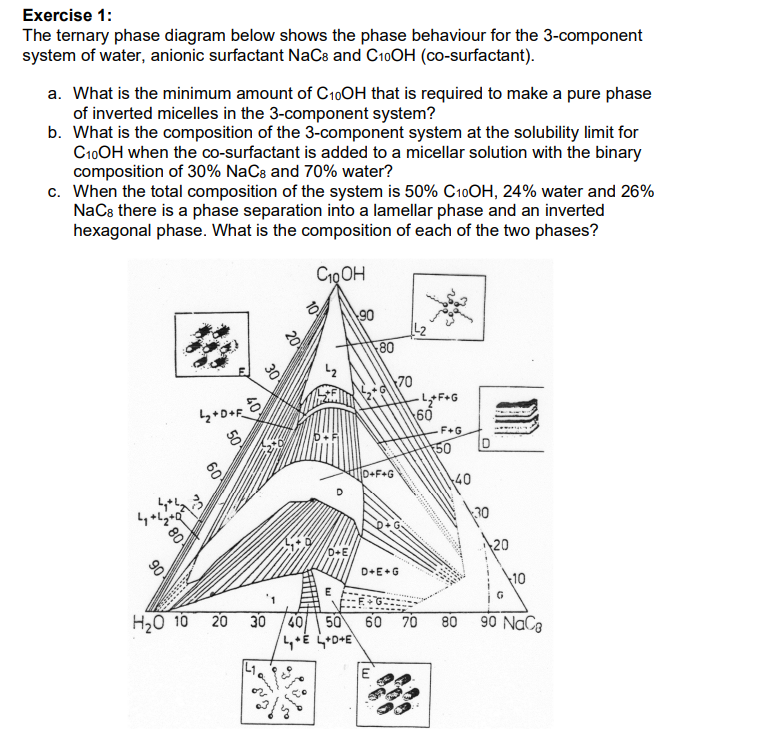
Exercise 1: The ternary phase diagram below shows the phase behaviour for the 3-component system of water, anionic surfactant NaC8 and C10OH (co-surfactant). a. What is the minimum amount of C10OH that is required to make a pure phase of inverted micelles in the 3-component system? b. What is the composition of the 3-component system at the solubility limit for C10OH when the co-surfactant is added to a micellar solution with the binary composition of 30%NaC8 and 70% water? c. When the total composition of the system is 50%C10OH,24% water and 26% NaC8 there is a phase separation into a lamellar phase and an inverted hexagonal phase. What is the composition of each of the two phases? Exercise 1: The ternary phase diagram below shows the phase behaviour for the 3-component system of water, anionic surfactant NaC8 and C10OH (co-surfactant). a. What is the minimum amount of C10OH that is required to make a pure phase of inverted micelles in the 3-component system? b. What is the composition of the 3-component system at the solubility limit for C10OH when the co-surfactant is added to a micellar solution with the binary composition of 30%NaC8 and 70% water? c. When the total composition of the system is 50%C10OH,24% water and 26% NaC8 there is a phase separation into a lamellar phase and an inverted hexagonal phase. What is the composition of each of the two phases
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


