Question: Exercise 1.1. A particular element crystallises in the FCC structure with a single atom basis. The cubic lattice constant (or edge length of unit cell)
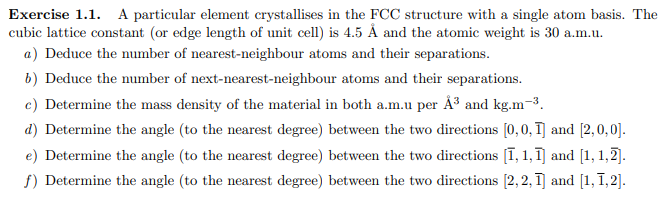
Exercise 1.1. A particular element crystallises in the FCC structure with a single atom basis. The cubic lattice constant (or edge length of unit cell) is 4.5A and the atomic weight is 30 a.m.u. a) Deduce the number of nearest-neighbour atoms and their separations. b ) Deduce the number of next-nearest-neighbour atoms and their separations. c) Determine the mass density of the material in both a.m.u per A3 and kgm3. d) Determine the angle (to the nearest degree) between the two directions [0,0,1] and [2,0,0]. e) Determine the angle (to the nearest degree) between the two directions [1,1,1] and [1,1,2]. f) Determine the angle (to the nearest degree) between the two directions [2,2,1] and [1,1,2]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


