Question: Exercise 11. Consider a hydrogen-oxygen fuel cell. a) What volume of H2(g), stored at 25C at a pressure of 155 atm, would be needed
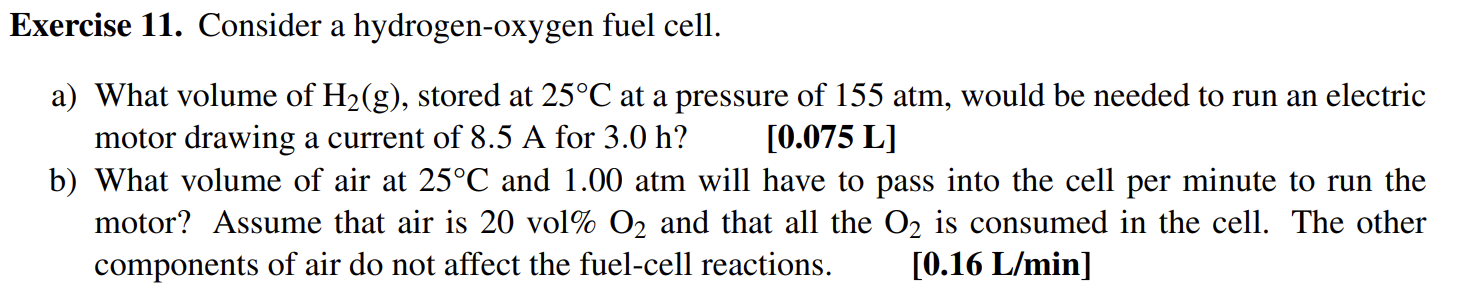
Exercise 11. Consider a hydrogen-oxygen fuel cell. a) What volume of H2(g), stored at 25C at a pressure of 155 atm, would be needed to run an electric motor drawing a current of 8.5 A for 3.0 h? [0.075 L] b) What volume of air at 25C and 1.00 atm will have to pass into the cell per minute to run the motor? Assume that air is 20 vol% O2 and that all the O2 is consumed in the cell. The other components of air do not affect the fuel-cell reactions. [0.16 L/min]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


