Question: EXERCISE 2 . 1 8 ( 1 ) Write the resonance structures of the following ions. ( 2 ) State which structure ( if any
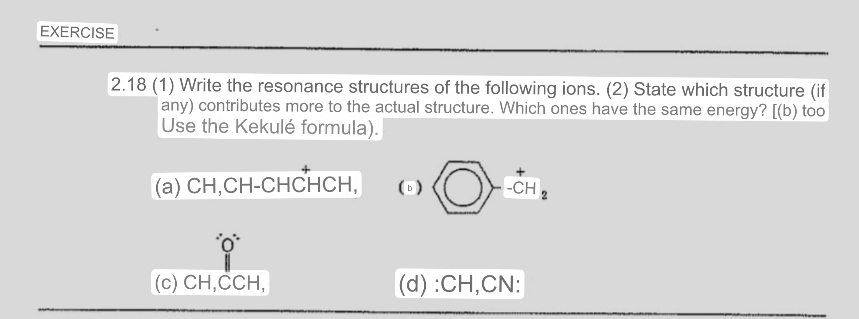
EXERCISE
Write the resonance structures of the following ions. State which structure if any contributes more to the actual structure. Which ones have the same energy? b too Use the Kekul formula
a
b
c
d : :
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


