Question: Exercise 2: Distillation Cut Points A professional engineer will receive 110 million barrels per day (M B/D) of crude oil to produce 15M B/D of
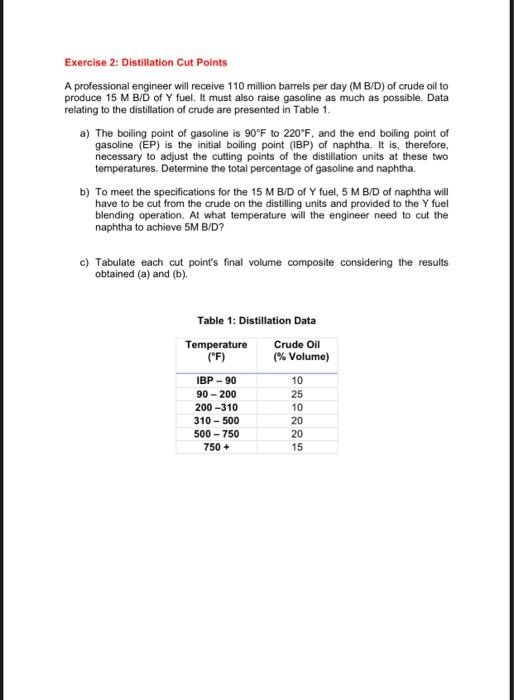
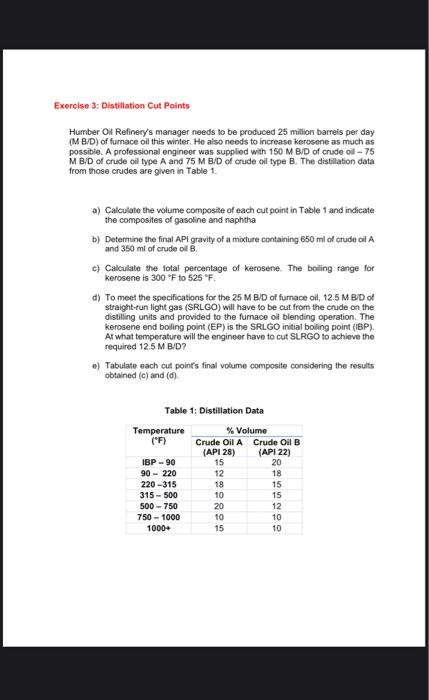

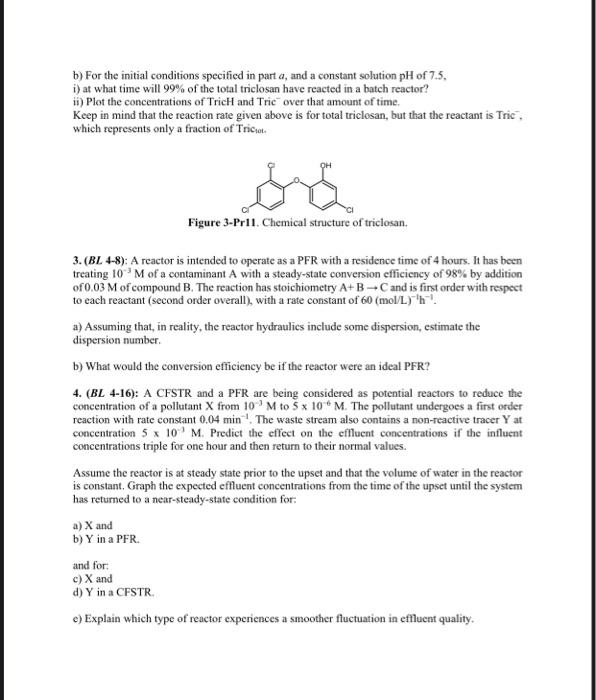
Exercise 2: Distillation Cut Points A professional engineer will receive 110 million barrels per day (M B/D) of crude oil to produce 15M B/D of Y fuel. It must also raise gasoline as much as possible. Data relating to the distillation of crude are presented in Table 1. a) The boiling point of gasoline is 90F to 220F, and the end boiling point of gasoline (EP) is the initial boiling point (IBP) of naphtha. It is, therefore, necessary to adjust the cutting points of the distillation units at these two temperatures. Determine the total percentage of gasoline and naphtha. b) To meet the specifications for the 15MB/D of Y fuel, 5MB/D of naphtha will have to be cut from the crude on the distilling units and provided to the Y fuel blending operation. At what temperature will the engineer need to cut the naphtha to achieve 5M B/D? c) Tabulate each cut point's final volume composite considering the results obtained (a) and (b). Table 1: Distillation Data Humber Oil Refinery's manager needs to be produced 25 million barreis per day (M BVD) of fumace oil this winter. He also needs to increase kerosene as much as possible. A professional engineer was supplied with 150MBVD of crude oil - 75 MB/D of crude oil type A and 75M B/D of crude oil type B. The distillation data from those crudes are given in Table 1. a) Calculate the volume composite of each cut point in Table 1 and indicate the composites of gascline and naphtha b) Determine the final API gravity of a mixture containing 650ml of crude oil A and 350ml of crude cil 8 . c) Calculate the fotal percentage of kerosene. The boiling range for kerosene is 300F to 525F. d) To meet the specifications for the 25M BID of furnace oil, 12.5MBD of straight-run light gas (SRLGD) will have to be cut from the crude on the distiling units and provided to the furnace oi blending operation. The kerosene end boling point (EP) is the SRLGO initial boling point (IBP). At what temperature will the engineor have to cut SLRGO to achieve the required 12.5M BID? e) Tabulate each cut point's final volume composite considering the results obtained (c) and (d). Table 1: Distillation Data 1. (BL31) : a) Identify the order of the reaction with respect to i) reactant A, ii) reactant B, and iii) overall in the rate expression: rA=kCA0.5CB. b) The reaction whose kinetics is expressed in part (a) has the stoichiometry: 21A+BP What is the order of the reaction order with respect to i) reactant A, ii) reactant B, and iii) overall if the stoichiometry is re-written as: A+2B2P c) For biological degradation of substrate S by microorganisms at concentration X, the rate of degradation is often expressed as follows: rS=kXS/(KS+S). Identify-if possible-the order of the reaction with respect to i) the microorganism concentration, ii) the substrate concentration, and iii) the overall order. d) In some engineered systems for biological degradation, the substrate concentration is very low, so that SKs. In that circumstance, find the order of the reaction with respect to i) the microorganism concentration, ii) the substrate concentration, and iii) the overall order. 2. (BL 3-11): Triclosan is an anti-microbial agent that can react with chlorine during drinking water or wastewater disinfection. This reaction depends on solution pH, because both triclosan (TricH) and the form of chlorine used in disinfection (HOCl) are weak acids. The acid dissociation reactions and constants for HOCl and TricH are as follows: HOClOCl+H+logKa=7.55TricHTric+H+logKa=7.9 The reaction between HOCl and Tric proceeds much more rapidly than that between HOCl and TricH, or between OCland either TricH or Tric . The dominant reaction is first order in both HOCl and Tric , with a rate constant of k=5.4103(mol/L)1s1. The rate of disappearance of total triclosan is therefore: rTrictot=[5.4+103(molsL)](CTric-)(CHOCl) a) For a solution containing 0.5mol/L total triclosan and 14mol/L total OCl, plot the initial rate of triclosan reaction ( mol/Ls ) over the pH range 4-11. Use a logarithmic scale for the rate. Hint: Recall that for a monoprotic acid HAH++A,0=[H+]+Ka[H+] and 1=[H+]+KaKa where n is the fraction of the total acid that as lost n protons. b) For the initial conditions specified in part a, and a constant solution pH of 7.5, i) at what time will 99% of the total triclosan have reacted in a batch reactor? ii) Plot the concentrations of TricH and Tric over that amount of time. Keep in mind that the reaction rate given above is for total triclosan, but that the reactant is Tric-. which represents only a fraction of Triciot. Figure 3-Pr11. Chemical structure of triclosan. 3. (BL 4-8): A reactor is intended to operate as a PFR with a residence time of 4 hours. It has been treating 103M of a contaminant A with a steady-state conversion efficiency of 98% by addition of 0.03M of compound B. The reaction has stoichiometry A+BC and is first order with respect to each reactant (second order overall), with a rate constant of 60(mol/L)1h1. a) Assuming that, in reality, the reactor hydraulics include some dispersion, estimate the dispersion number. b) What would the conversion efficiency be if the reactor were an ideal PFR? 4. (BL 4-16): A CFSTR and a PFR are being considered as potential reactors to reduce the concentration of a pollutant X from 103M to 5106M. The pollatant undergoes a first order reaction with rate constant 0.04min1. The waste stream also contains a non-reactive tracer Y at concentration 5103M. Predict the effect on the effluent concentrations if the influent concentrations triple for one hour and then return to their normal values. Assume the reactor is at steady state prior to the upset and that the volume of water in the reactor is constant. Graph the expected effluent concentrations from the time of the upset until the system has returned to a near-steady-state condition for: a) X and b) Y in a PFR. and for: c) X and d) Y in a CFSTR. e) Explain which type of reactor experiences a smoother fluctuation in effluent quality
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


