Question: Exercise 2 Maxwell's relations (MRs) are equalities relating partial derivatives of thermodynamic potentials: U, H, A, G with respect to thermodynamic variables: P, V, T,
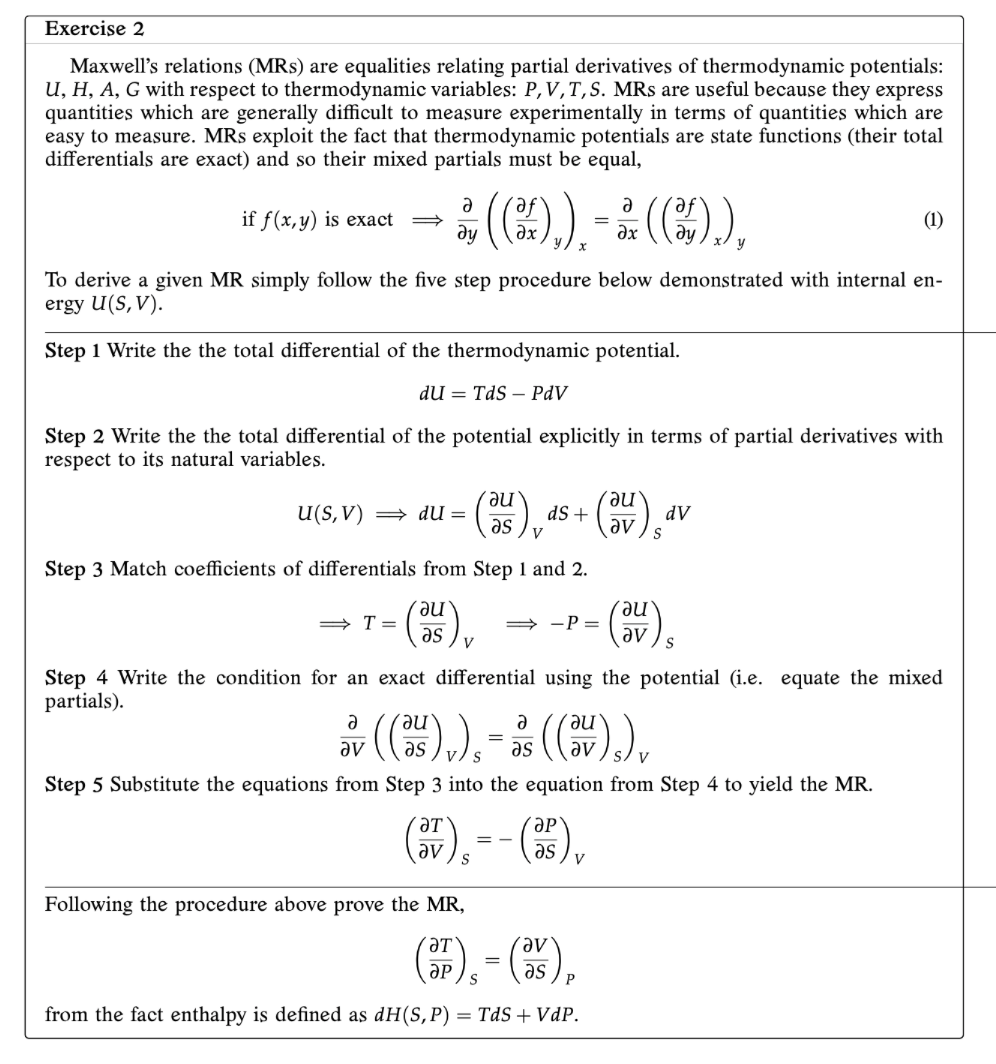
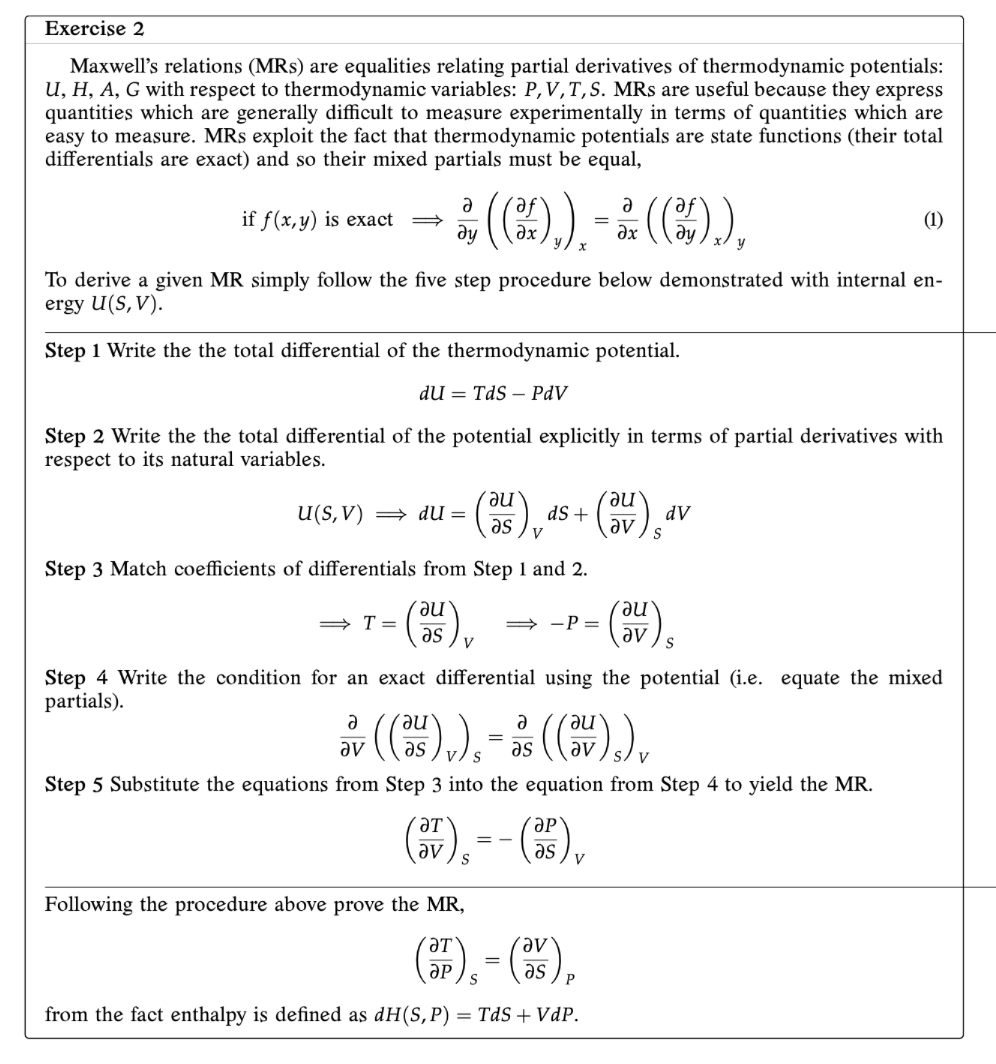
Exercise 2 Maxwell's relations (MRs) are equalities relating partial derivatives of thermodynamic potentials: U, H, A, G with respect to thermodynamic variables: P, V, T, S. MRs are useful because they express quantities which are generally difficult to measure experimentally in terms of quantities which are easy to measure. MRs exploit the fact that thermodynamic potentials are state functions (their total differentials are exact) and so their mixed partials must be equal, if f (x, y) is exact of ( (Ox ) , ) , = BE ( ( 85 ) ) , (1) To derive a given MR simply follow the five step procedure below demonstrated with internal en- ergy U(S, V). Step 1 Write the the total differential of the thermodynamic potential. du = Tds - PdV Step 2 Write the the total differential of the potential explicitly in terms of partial derivatives with respect to its natural variables. u(s, V) = du = (ne) as + rau as v (av dv Step 3 Match coefficients of differentials from Step 1 and 2. au as av) Step 4 Write the condition for an exact differential using the potential (i.e. equate the mixed partials). or (( 's ) v ), = as ( ( ov ) s ) v Step 5 Substitute the equations from Step 3 into the equation from Step 4 to yield the MR. av ) s ap as ) v Following the procedure above prove the MR, from the fact enthalpy is defined as dH(S, P) = Tds + VdP
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


