Question: Exercise #2: Vapor-Liquid Equilibria (VLE) data for the ethanol (1) - benzene (2) system at 40C are presented below: The vapor pressures of the pure
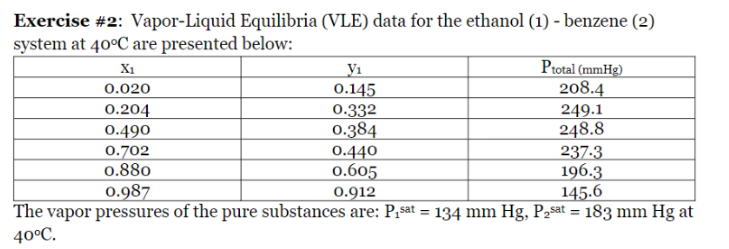

Exercise \#2: Vapor-Liquid Equilibria (VLE) data for the ethanol (1) - benzene (2) system at 40C are presented below: The vapor pressures of the pure substances are: P1 sat =134mmHg,P2sat=183mmHg at 40C. - Using the van Laar equation, compare the predicted and experimental values of total pressure and y1 at x1=0.490. Comment on the accuracy of the van Laar equation compared to the data, and recommend a different activity coefficient model. - Show if these data thermodynamically consistent. - Is there an azeotrope, where? - Explain how to reproduce these data using an appropriate fugacity model, and outline the solution. Exercise \#2: Vapor-Liquid Equilibria (VLE) data for the ethanol (1) - benzene (2) system at 40C are presented below: The vapor pressures of the pure substances are: P1 sat =134mmHg,P2sat=183mmHg at 40C. - Using the van Laar equation, compare the predicted and experimental values of total pressure and y1 at x1=0.490. Comment on the accuracy of the van Laar equation compared to the data, and recommend a different activity coefficient model. - Show if these data thermodynamically consistent. - Is there an azeotrope, where? - Explain how to reproduce these data using an appropriate fugacity model, and outline the solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


