Question: Exercise #3 : (40 points) A binary liquid mixture @70 C has these activity coefficients: The pure component vapor pressures at this temperature are P1sat=0.03107MPa
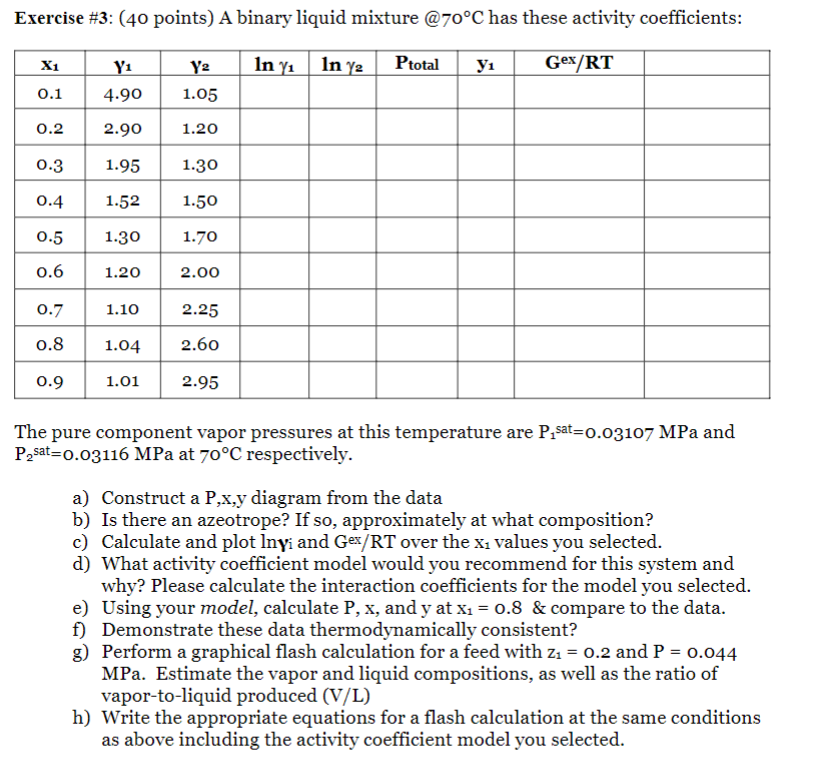
Exercise #3 : (40 points) A binary liquid mixture @70 C has these activity coefficients: The pure component vapor pressures at this temperature are P1sat=0.03107MPa and P2sat=0.03116MPa at 70C respectively. a) Construct a P,x,y diagram from the data b) Is there an azeotrope? If so, approximately at what composition? c) Calculate and plot ln yi and G ex/RT over the x1 values you selected. d) What activity coefficient model would you recommend for this system and why? Please calculate the interaction coefficients for the model you selected. e) Using your model, calculate P,x, and y at x1=0.8 \& compare to the data. f) Demonstrate these data thermodynamically consistent? g) Perform a graphical flash calculation for a feed with z1=0.2 and P=0.044 MPa. Estimate the vapor and liquid compositions, as well as the ratio of vapor-to-liquid produced (V/L) h) Write the appropriate equations for a flash calculation at the same conditions as above including the activity coefficient model you selected. Exercise #3 : (40 points) A binary liquid mixture @70 C has these activity coefficients: The pure component vapor pressures at this temperature are P1sat=0.03107MPa and P2sat=0.03116MPa at 70C respectively. a) Construct a P,x,y diagram from the data b) Is there an azeotrope? If so, approximately at what composition? c) Calculate and plot ln yi and G ex/RT over the x1 values you selected. d) What activity coefficient model would you recommend for this system and why? Please calculate the interaction coefficients for the model you selected. e) Using your model, calculate P,x, and y at x1=0.8 \& compare to the data. f) Demonstrate these data thermodynamically consistent? g) Perform a graphical flash calculation for a feed with z1=0.2 and P=0.044 MPa. Estimate the vapor and liquid compositions, as well as the ratio of vapor-to-liquid produced (V/L) h) Write the appropriate equations for a flash calculation at the same conditions as above including the activity coefficient model you selected
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


