Question: * EXERCISE: Explain why Mo(PMe3) 3H2 is a di-hydride (contains two separate H ligands), but Mo(CO);(PR3)2(Hz) contains the dihydrogen ligand (Me = methyl, R =
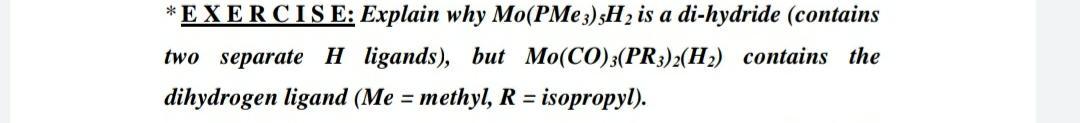
* EXERCISE: Explain why Mo(PMe3) 3H2 is a di-hydride (contains two separate H ligands), but Mo(CO);(PR3)2(Hz) contains the dihydrogen ligand (Me = methyl, R = isopropyl)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


