Question: Exothermic gas phase reaction (previous CHE 430 exam problem; partial). A reversible, exothermic, non-catalytic, gas-phase reaction N2O22NO (A) (B) with a reaction rate of rA=k(CAKeqCB)
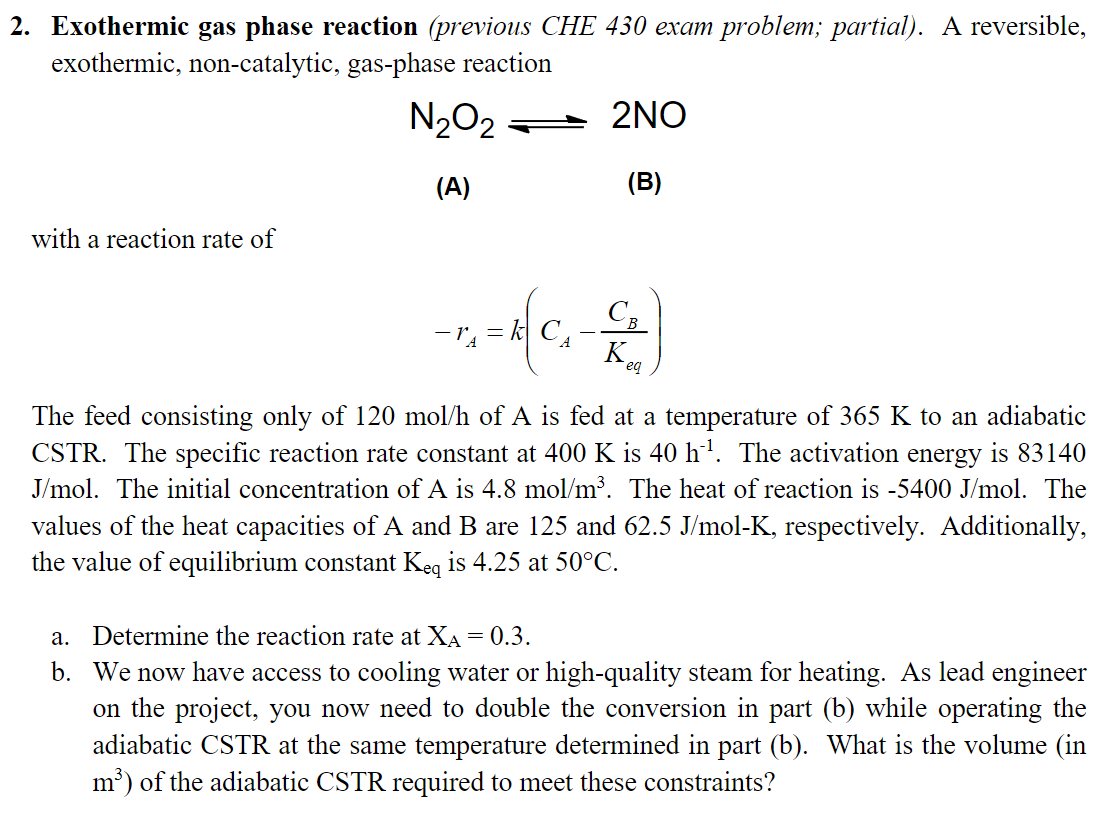
Exothermic gas phase reaction (previous CHE 430 exam problem; partial). A reversible, exothermic, non-catalytic, gas-phase reaction N2O22NO (A) (B) with a reaction rate of rA=k(CAKeqCB) The feed consisting only of 120mol/h of A is fed at a temperature of 365K to an adiabatic CSTR. The specific reaction rate constant at 400K is 40h1. The activation energy is 83140 J/mol. The initial concentration of A is 4.8mol/m3. The heat of reaction is 5400J/mol. The values of the heat capacities of A and B are 125 and 62.5J/molK, respectively. Additionally, the value of equilibrium constant Keq is 4.25 at 50C. a. Determine the reaction rate at XA=0.3. b. We now have access to cooling water or high-quality steam for heating. As lead engineer on the project, you now need to double the conversion in part (b) while operating the adiabatic CSTR at the same temperature determined in part (b). What is the volume (in m3 ) of the adiabatic CSTR required to meet these constraints? Exothermic gas phase reaction (previous CHE 430 exam problem; partial). A reversible, exothermic, non-catalytic, gas-phase reaction N2O22NO (A) (B) with a reaction rate of rA=k(CAKeqCB) The feed consisting only of 120mol/h of A is fed at a temperature of 365K to an adiabatic CSTR. The specific reaction rate constant at 400K is 40h1. The activation energy is 83140 J/mol. The initial concentration of A is 4.8mol/m3. The heat of reaction is 5400J/mol. The values of the heat capacities of A and B are 125 and 62.5J/molK, respectively. Additionally, the value of equilibrium constant Keq is 4.25 at 50C. a. Determine the reaction rate at XA=0.3. b. We now have access to cooling water or high-quality steam for heating. As lead engineer on the project, you now need to double the conversion in part (b) while operating the adiabatic CSTR at the same temperature determined in part (b). What is the volume (in m3 ) of the adiabatic CSTR required to meet these constraints
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


