Question: exp,ain Consider the reaction 2X - Y+Z @ Macmillan Learning When the concentration of X is doubled, the reaction rate increases by a factor of
exp,ain
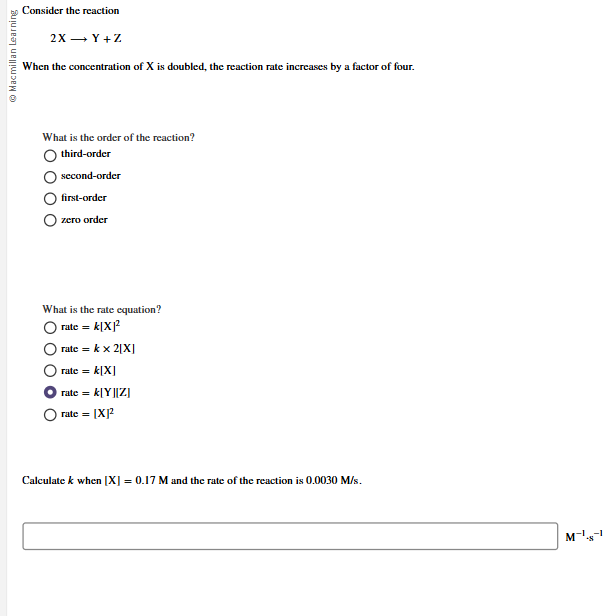 Consider the reaction 2X - Y+Z @ Macmillan Learning When the concentration of X is doubled, the reaction rate increases by a factor of four. What is the order of the reaction? O third-order second-order O first-order O zero order What is the rate equation? O rate = k[X] O rate = k x 2[X] O rate = k[X] O rate = k|Y]IZI O rate = [X]? Calculate
Consider the reaction 2X - Y+Z @ Macmillan Learning When the concentration of X is doubled, the reaction rate increases by a factor of four. What is the order of the reaction? O third-order second-order O first-order O zero order What is the rate equation? O rate = k[X] O rate = k x 2[X] O rate = k[X] O rate = k|Y]IZI O rate = [X]? Calculate Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


