Question: Experiment 2 : Heat Capactiy Experiment 2 : Heat capacity i want results 1. Calculate the weight of the cold distilled water in gram: Wc=WcalteWcal
Experiment 2 : Heat Capactiy
Experiment 2 : Heat capacity i want results
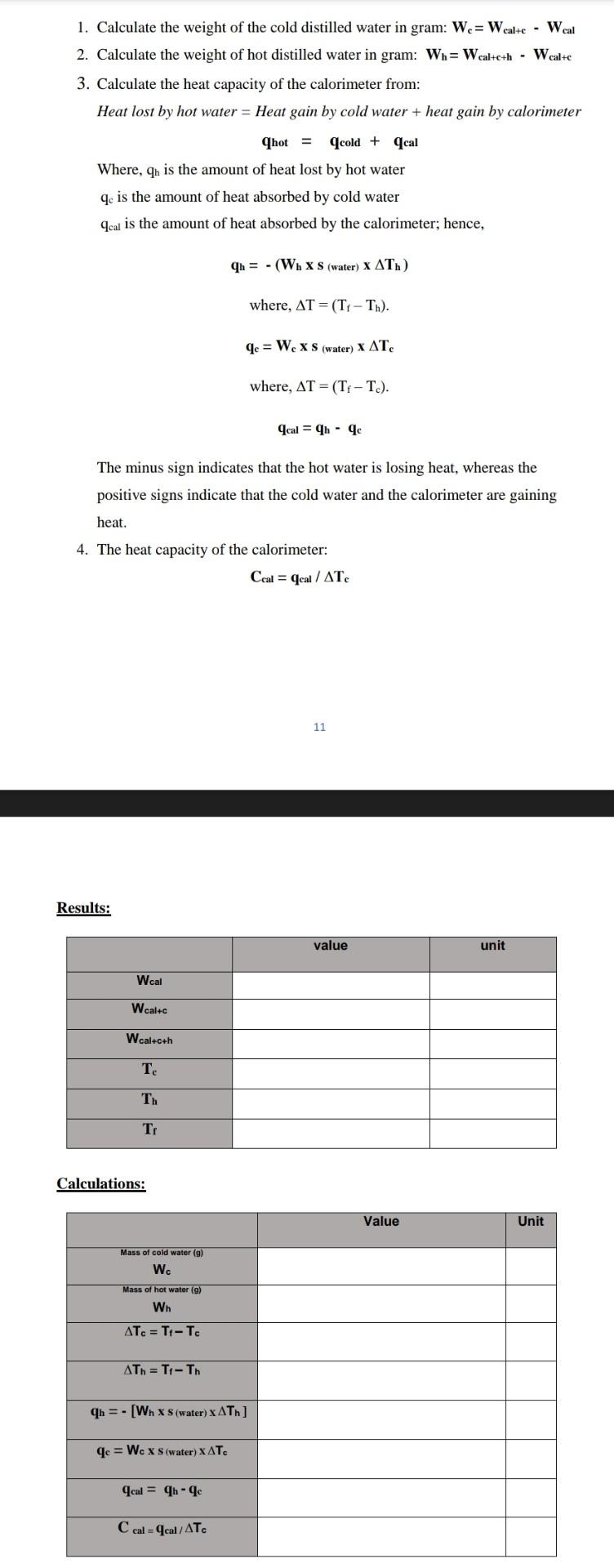
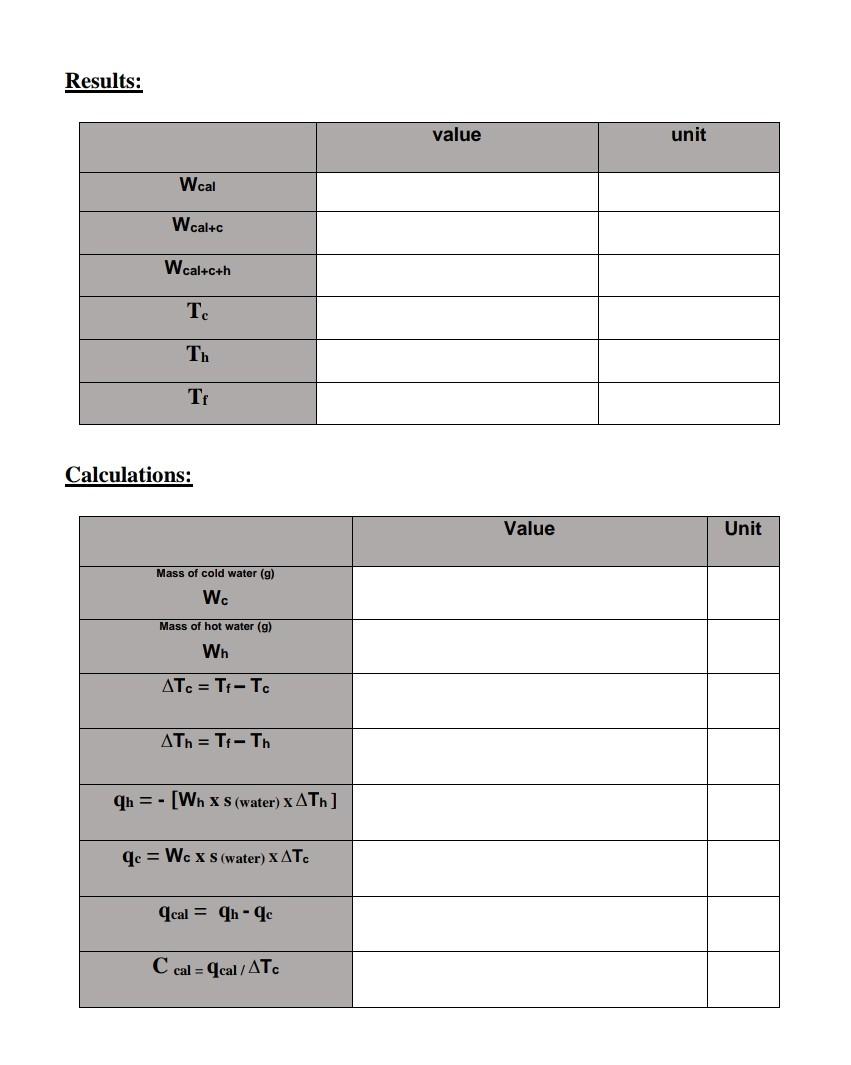
1. Calculate the weight of the cold distilled water in gram: Wc=WcalteWcal 2. Calculate the weight of hot distilled water in gram: Wh=Wcalte+hWcalte 3. Calculate the heat capacity of the calorimeter from: Heat lost by hot water = Heat gain by cold water + heat gain by calorimeter qhot=qcold+qcal Where, qb is the amount of heat lost by hot water qc is the amount of heat absorbed by cold water qcal is the amount of heat absorbed by the calorimeter; hence, qh=(Whs(water)xTh)where,T=(TfTh).qc=Wcxs(water)Tcwhere,T=(TfTc).qcal=qhqc The minus sign indicates that the hot water is losing heat, whereas the positive signs indicate that the cold water and the calorimeter are gaining heat. 4. The heat capacity of the calorimeter: Ccal=qcal/Tc 11 Results: Calculations: Results: Calculations: 1. Calculate the weight of the cold distilled water in gram: Wc=WcalteWcal 2. Calculate the weight of hot distilled water in gram: Wh=Wcalte+hWcalte 3. Calculate the heat capacity of the calorimeter from: Heat lost by hot water = Heat gain by cold water + heat gain by calorimeter qhot=qcold+qcal Where, qb is the amount of heat lost by hot water qc is the amount of heat absorbed by cold water qcal is the amount of heat absorbed by the calorimeter; hence, qh=(Whs(water)xTh)where,T=(TfTh).qc=Wcxs(water)Tcwhere,T=(TfTc).qcal=qhqc The minus sign indicates that the hot water is losing heat, whereas the positive signs indicate that the cold water and the calorimeter are gaining heat. 4. The heat capacity of the calorimeter: Ccal=qcal/Tc 11 Results: Calculations: Results: Calculations
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


