Question: Experiment: UV-vis experiment was done to determine the unknown concentration of an orga compound solution. First, a stock solution of Compound A (Molar Mass 893.5
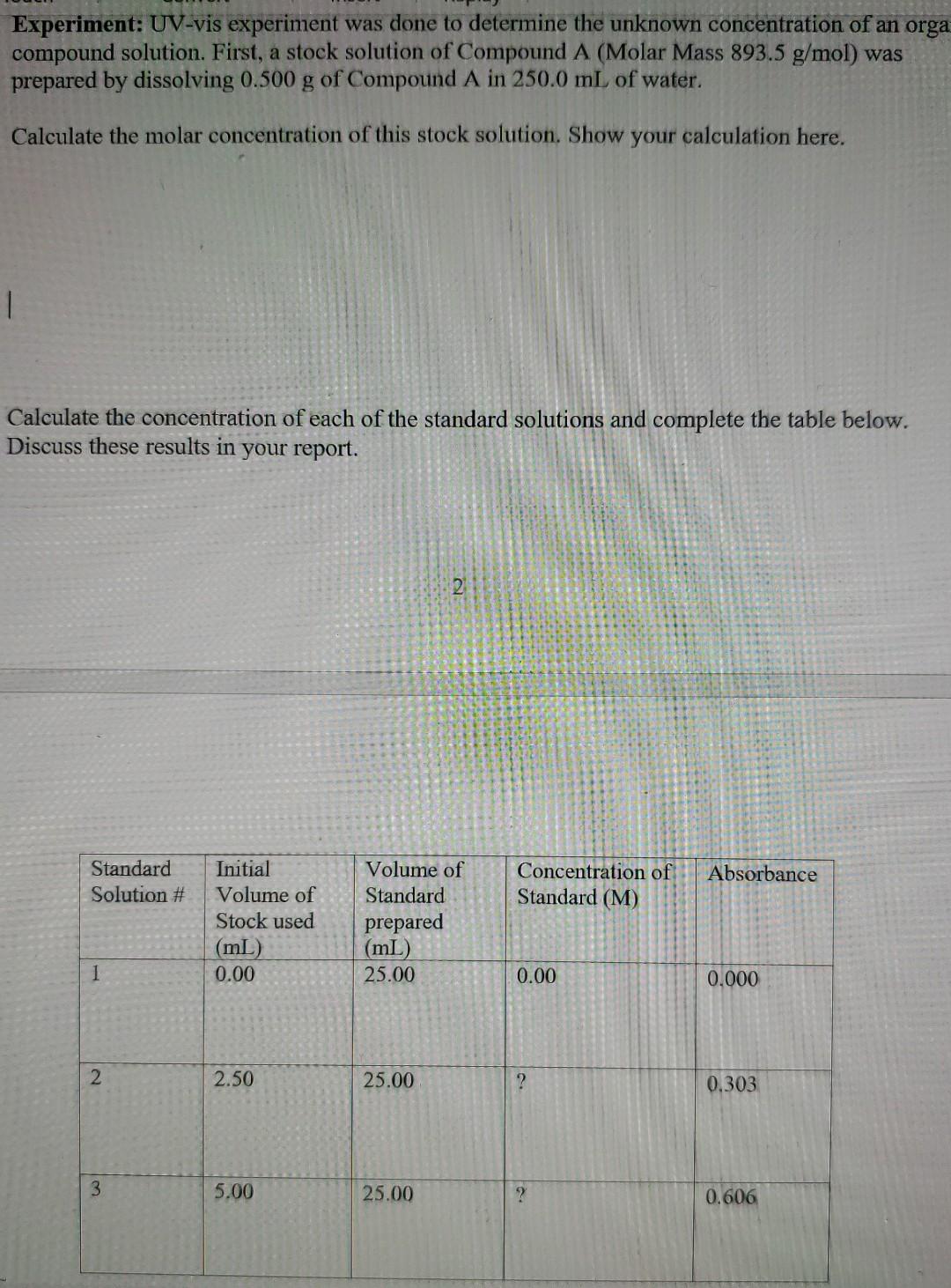
Experiment: UV-vis experiment was done to determine the unknown concentration of an orga compound solution. First, a stock solution of Compound A (Molar Mass 893.5 g/mol) was prepared by dissolving 0.500 g of Compound A in 250.0 mL of water. Calculate the molar concentration of this stock solution. Show your calculation here. 1 Calculate the concentration of each of the standard solutions and complete the table below. Discuss these results in your report. 2 Standard Solution # Concentration of Standard (M) Absorbance Initial Volume of Stock used (mL) 0.00 Volume of Standard prepared (mL) 25.00 1 0.00 0.000 2 2.50 25.00 ? 0.303 3 5.00 25.00 0.606
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


