Question: explain Decide which intermolecular forces act between the molecules of each compound in the table below. intermolecular forces (check all that apply) compound dispersion dipole
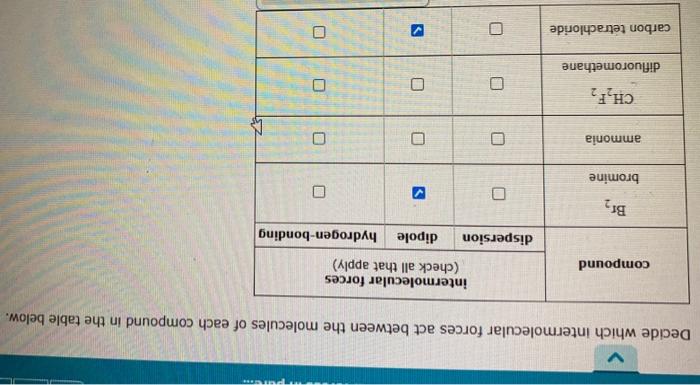
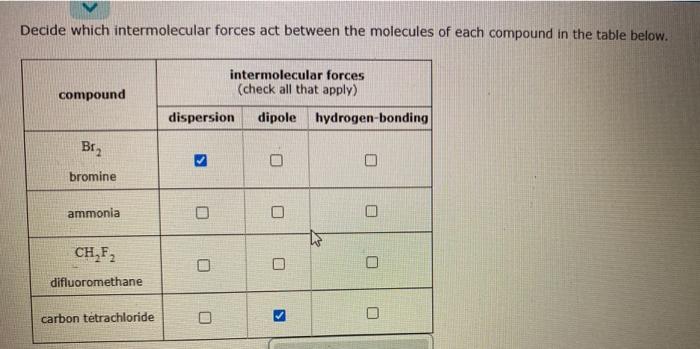
Decide which intermolecular forces act between the molecules of each compound in the table below. intermolecular forces (check all that apply) compound dispersion dipole hydrogen-bonding Br2 bromine ammonia O CH,F2 difluoromethane carbon tetrachloride U Decide which intermolecular forces act between the molecules of each compound in the table below. intermolecular forces (check all that apply) compound dispersion dipole hydrogen-bonding Br2 O bromine ammonia ET CH,F2 O O difluoromethane carbon tetrachloride
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


