Question: A fuel is burned in 20% excess air. Determine the percent by volume of each constituent in the flue gas. The fuel has the
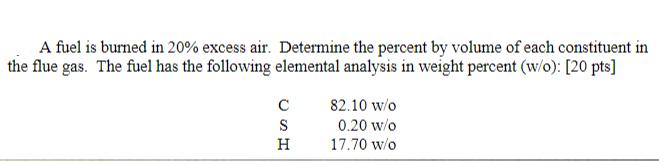
A fuel is burned in 20% excess air. Determine the percent by volume of each constituent in the flue gas. The fuel has the following elemental analysis in weight percent (w/o): [20 pts] C SH 82.10 w/o 0.20 w/o 17.70 w/o
Step by Step Solution
3.32 Rating (152 Votes )
There are 3 Steps involved in it
Sure lets work through the question step by step 1 Calculate the moles of each element Carbon C Mola... View full answer

Get step-by-step solutions from verified subject matter experts


