Question: Explain in details pleas 3. Locate the radial and angular nodes in the hydrogen atom orbitals represented by wgpx and 1,031,: where: (1)3x2( r r2)
Explain in details pleas
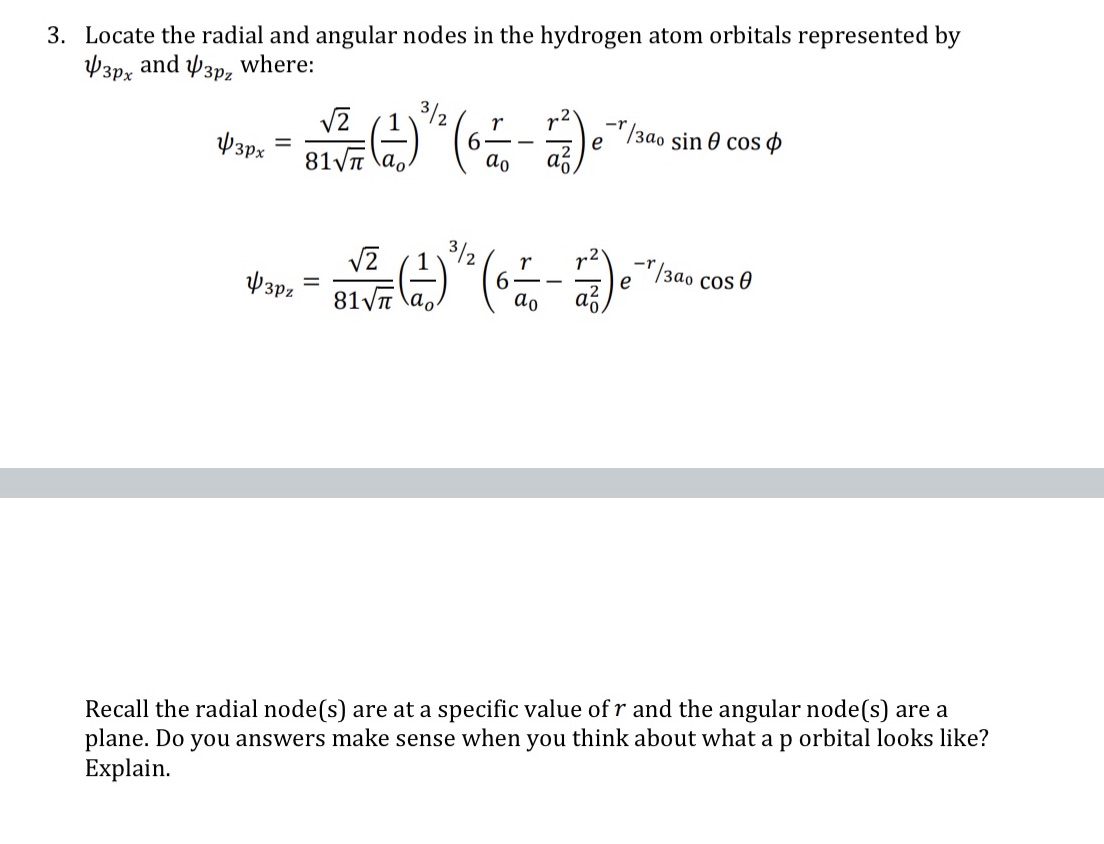
3. Locate the radial and angular nodes in the hydrogen atom orbitals represented by wgpx and 1,031,: where: (1)3x2( r r2) = 6 /3otrsin9COS 11'3\" 81% a0 a0 a3 3/ 2 $3 = '5 (i) 2 6L_r hacose \"2 81% a0 a0 a5 Recall the radial node[s] are at a specific value of r and the angular node[s] are a plane. Do you answers make sense when you think about what a p orbital looks like? Explain
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


